By Jennifer Bogdan, P.Ag., CCA

Source: Saskatchewan Ministry of Agriculture
Aphanomyces root rot, commonly referred to as part of root rot complex, poses an economic threat to pea and lentil production in Saskatchewan. Although it was only positively identified in the province in 2012, the pathogen likely has been present for much longer. Aphanomyces root rot causes severe damage to the roots, causing infected plants to wilt and die prematurely. In wet years, yield loss in peas has been observed over 70 per cent under high Aphanomyces root rot infections. Long-lived resting spores, the absence of genetic resistance, and the lack of control options make Aphanomyces root rot a difficult disease to manage.
Host Crops
Aphanomyces root rot is caused by Aphanomyces euteiches Drechs, a highly specialized pathogen of legumes and pulses. While this pathogen has a number of legume host plants, peas and lentils are the most susceptible pulse crops to infection. Faba beans and sainfoin exhibit good partial (quantitative) resistance to Aphanomyces, and chickpeas are considered moderately resistant. Soybeans and fenugreek are both non-host crops to A. euteiches. Susceptibility of dry beans and alfalfa to Aphanomyces root rot infection varies among the different varieties. Cicer milkvetch is also very susceptible to infection by Aphanomyces.
Life Cycle, Symptoms, and Crop Impact
Aphanomyces euteiches is classified as an oomycete, or water mould, and is not a true fungus. The resting spores, called oospores, are thick-walled and allow the pathogen to survive in the soil during harsh winter conditions.
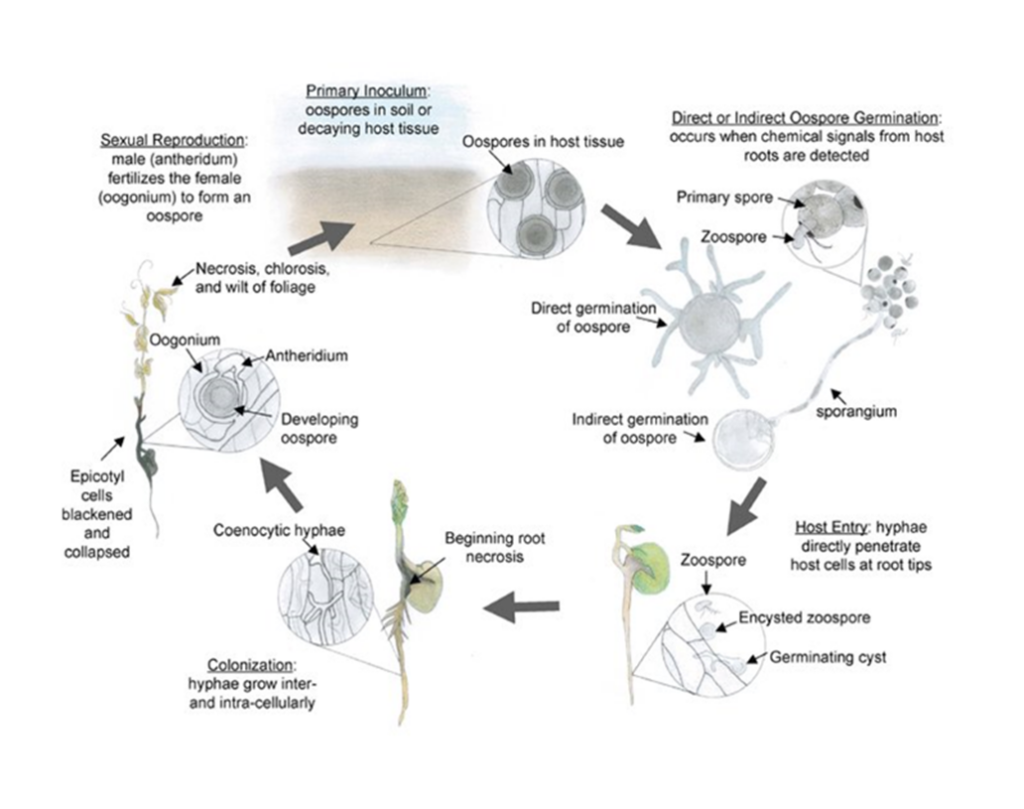
Source: Hughes and Grau (2007), courtesy of the American Phytopathological Society

Source: Syama Chatterton, Agriculture and Agri-Food Canada
In the presence of host plant roots, the oospores undergo either direct germination or indirect germination. Oospores can germinate to produce vegetative hyphae that can directly infect the plant root at any time throughout the growing season. Oospores can also germinate indirectly to form sporangia which release mobile zoospores. These zoospores are able to detect chemical signals in root exudates from suitable host plants, and swim through water-filled pores toward the roots where hyphae are produced and infection takes place. Hyphae spread throughout the root tissue, then into the below-ground stem, and eventually the entire root system is colonized. A few days after infection, the pathogen enters its sexual stage and produces oospores, which can survive in the soil for over 10 years, and are the primary source of inoculum for future infections in susceptible crops. Oospore production can occur in as little as 10 to 14 days after the first root infection.
Aphanomyces has also been found to spread from the roots of a single infected plant to the roots of healthy neighbouring plants up to 18 centimetres (cm) away.
Variability within A. euteiches populations continues to be explored. French scientists have identified 11 virulence types from 109 isolates tested, with virulence type I being the most predominant, the most aggressive, and causing the most severe symptoms in peas. The University of Alberta tested eight isolates from Alberta and Manitoba and found the majority of strains to be virulence type I, with one strain classified as virulence type III. The University of Saskatchewan is currently testing 35 isolates from Saskatchewan and Alberta. Initial observations have found that there is a range of virulence among the isolates, including some that are very virulent, but full analysis has not yet been completed.
Roots of host plants infected with Aphanomyces root rot become soft and have a water-soaked, honey-brown or caramel-coloured appearance. Infections begin on the lateral roots, then spread to the main root, and eventually into the epicotyl, stopping at where the green stem tissue begins. At this time, infected plants start to turn pale in colour and are especially noticeable when beside healthy plants. Yellow areas in the crop may begin to appear, although distinct patches are not always seen, and it is possible to see healthy plants right beside infected plants. As the season progresses and conditions remain favourable, the main root typically becomes colonized by other pathogens and turns dark brown to black.

Source: Syama Chatterton, Agriculture and Agri-Food Canada

Source: Syama Chatterton, Agriculture and Agri-Food Canada
The most severe symptoms and crop yellowing are seen when Aphanomyces is present alongside other pathogens, with Fusarium spp being the most common. Symptoms of Aphanomyces root rot are not always clear-cut because in most cases, a pathogen complex exists. It can be difficult to see the honey-brown root discolouration of Aphanomyces when Fusarium is also present. At advanced Aphanomyces root rot infection stages, only the vascular bundles remain, as the entire root system has decayed and plants are chlorotic, wilted, and will prematurely die. Aphanomyces symptoms appear within 7-14 days after the first root infection, depending on soil moisture and temperature, and susceptible host plants can become infected at any growth stage. Even though root infections are favoured by wet conditions, the symptoms and yield impact are most severe under warm, dry conditions after infection takes place.

Source: Jennifer Bogdan

Source: Jennifer Bogdan
Severe root rot caused by Aphanomyces impairs the uptake of water and nutrients by infected plants. Yield loss from Aphanomyces root rot can be difficult to assess because of the numerous indirect effects it has on the crop, as well as the timing of the initial infection. Early season infections cause plants to die prematurely, causing a direct and substantial yield loss. Later season infections can delay crop maturity, thereby affecting harvest timing, and also weaken the plant stems, causing severe lodging and harvest challenges. Research has reported reductions in both pod size and seed number, as well as decreased seed quality due to Aphanomyces root rot. A thin crop stand can also cause challenges in weed management as weed competition can negatively impact yield, affect harvestability, cause increased dockage at the elevator, and contribute to the weed seed bank in the soil.

Source: Syama Chatterton, Agriculture and Agri-Food Canada
Favourable Conditions
High soil moisture is a key factor in the development of Aphanomyces root rot. Soil moisture is critical in order for the zoospores to swim, via moisture films surrounding the soil particles, to the roots where initial infection takes place. High soil moisture caused from excessive rainfall, poor soil drainage, soil compaction, and/or heavy clay soils (35-40 per cent clay content) will favour Aphanomyces root rot development, although it can occur in any soil type. Research has suggested that Aphanomyces infection can occur at 45 per cent of the soil saturation level or higher, and the severity of the infection increases as soil moisture increases, although the moisture level at which maximum disease severity is reached is not known. Warm soil temperatures and the amount of disease inoculum are also contributing components of Aphanomyces root rot. The optimal soil temperature is 16°C for root infections, and 20 to 28°C for disease development, although if the soil is conducive for host plant growth, then Aphanomyces root rot can occur.
Aphanomyces infections can still occur in drier weather in fields with high disease inoculum. One possible explanation for this is direct oospore germination does not require as much moisture as what zoospores need, and roots that come in contact with oospores can become infected by their hyphae. Another possibility is that zoospores are formed early when water is available and root infection takes place, but then under drier conditions the effects of Aphanomyces root rot are exacerbated due to low water availability.

Source: Jennifer Bogdan
Because soil is variable across a field, the presence of Aphanomyces can also vary across a field. Patches of Aphanomyces root rot can develop in low spots that remain more saturated, or in compacted areas such as at field entrances. In some cases, patches are not always obvious. In years of excessive rainfall, it is possible to find nearly entire fields infected from Aphanomyces and other root rot pathogens. Within the soil profile, research found that the top 20 cm (eight inches) of soil contained the highest Aphanomyces inoculum levels, although there was still enough inoculum to cause infection as deep as 40-60 cm (16-24 inches).
Scouting and Damage Assessment
The key to scouting for Aphanomyces root rot (and other root rots) is to dig up plants and inspect the root systems. Above-ground portions of infected plants will still appear green initially. Only after the root system becomes more decayed will the stems and leaves gradually turn light green and then yellow. The unfortunate part of Aphanomyces root rot is that once infection and disease development take place, there is nothing that can be done to save the crop. If Aphanomyces is suspected, soil and/or root samples can be submitted for the DNA testing required for Aphanomyces euteiches confirmation. Knowing if Aphanomyces is present will help with disease management in future crop planning.
To soil test for Aphanomyces, remove the residue layer, then take a soil sample from the main rooting depth of six to eight inches, or less if the A-horizon is shallow. A minimum of two cups of soil is required for testing. A PCR (DNA) test is a quick test performed to detect the presence of oospores in the soil. Some labs are able to provide the number of oospores per gram of soil that can be used as a baseline for Aphanomyces root rot management. Soil sampling protocol testing conducted by SPG according to ongoing research conducted by Dr. Syama Chatterton has shown that utilizing the bait-test method is the most reliable way to detect and confirm positive Aphanomyces oospores, particularly in dry soil. A bait test not only involves planting a host plant in the soil sample but also involves a saturation incubation period that mimics the ideal conditions for an Aphanomyces infection to occur. Baiting is another technique offered by some labs that involves planting seeds of susceptible crops whose roots act as bait to attract Aphanomyces. The roots are then analyzed for the presence of oospores. Baiting is a slower process but can be more sensitive if low levels of Aphanomyces are present. Whole plant and root samples of suspect plants can also be tested for the presence of Aphanomyces. Some labs can also test for other pathogens such as Fusarium, Pythium, and Rhizoctonia at the same time. Refer to Testing for Aphanomyces Root Rot for more information on sampling, interpreting test results, and a listing of labs currently testing for Aphanomyces.
To measure root rot severity, researchers use a 0-5 Aphanomyces root rot rating scale, in combination with a 1-7 Fusarium root rot scale when both pathogens are present. Since most infections in the field are typically combined infections, the Fusarium 1-7 scale is often used, as it is very difficult to see the honey-brown discolouration from Aphanomyces when Fusarium is also present.

0 = No symptoms
3 = Start to see discolouration of crown/epicotyl. 50 to 75 per cent of the roots have browning
4 = Honey-brown epicotyl/crown area. 100 per cent of the roots are brown and first leaves are dying off
5 = Plant is dead. Decay and loss of all roots and usually only vascular string remains. Honey-brown epicotyl/crown area
Source: Syama Chatterton, Agriculture and Agri-Food Canada

1 = Pink nodules, no lesions on main tap root, and white-tan secondary roots
4 = Lesion encircles tap root with loss of nodules and some root
browning. Five to 10 per cent root mass reduction
7 = Tap root completely brown/black
Source: Syama Chatterton, Agriculture and Agri-Food Canada
Management
Crop Rotation and Weed Management

Source: Syama Chatterton, Agriculture and Agri-Food Canada
If a history of root rot exists, then individual fields should be tested for Aphanomyces euteiches. Fields confirmed to have A. euteiches present should not have peas or lentils planted on them for a minimum of six years, but preferably eight years. The best pulse crop options for these fields are faba beans, soybeans, or chickpeas, although chickpeas may develop low levels of infection. The challenge with Aphanomyces is that the oospores can survive in their resting state for 10-15 years, even when no host is present. Though inoculum levels will be reduced after a few years away from peas or lentils, fields with even moderate inoculum levels will require several years of non-host crops to reduce the inoculum to a level that will not impact yield. Since the effectiveness of crop rotation directly depends on the length of rotation, the longer the lapse between susceptible host crops, the more inoculum reduction that will occur in the soil, although complete eradication of Aphanomyces root rot through long-term rotations may not be achievable.
Though they are not crop hosts, weed host plants can also contribute to the amount of oospore inoculum in the soil. Volunteer alfalfa and white clover, shepherd’s-purse, chickweed, and field violet (field pansy) are all able to be infected by A. euteiches under the same wet conditions in which peas are infected. Diligent control of weedy host plants, in addition to crop rotation, is important for spore reduction in the soil.
Field Selection
Since high soil moisture is necessary to initiate disease development, planting peas and lentils on fields with lighter-textured (sandier) soils may help mitigate Aphanomyces root rot infections.
An interesting study in Sweden identified two groups of Aphanomyces root rot suppressive soils from soil samples taken at a 20 cm (8 inch) depth. The first group (S1) had high sand content (56 to 73 per cent), low clay content (9-12 per cent), and higher than average organic carbon content. The second group (S2) had high pH (greater than 6.7), high calcium content (greater than 17 centimoles per kilogram), and high clay content (19-21 per cent), with a higher ratio of vermiculite-smectite to illite-kaolinite, and therefore a higher cation exchange capacity.
In commercial fields of either S1 or S2 soils, a one-in-six-year pea rotation had not resulted in any infestation of Aphanomyces. Limited research on Prairie soils has been conducted, and no significant correlations have been found between soil properties and microbiome in fields with low or high disease severity. There are research projects underway studying possible connections between antagonistic bacteria and the soil microbiome on Aphanomyces root rot suppression.
Soil Amendments
Calcium is thought to prevent zoospore production from oospores, as soils with more free calcium had greater A. euteiches suppression and subsequent disease reduction. Research on the Prairies has begun in order to understand if liming soils will suppress Aphanomyces root rot, and if liming is both economically and physically feasible for growers. Preliminary results have indicated that surface applications of lime did not result in changes to the calcium concentration of the soil, and incorporation of high amounts of lime would likely be necessary.
Additional research is also being conducted to determine the effectiveness of Brassica green manure crops on Aphanomyces root rot suppression the year prior to planting a host crop, as some Brassica residues have been previously found to reduce Aphanomyces severity in other research studies.
Studies have found that fall incorporated oat residue from growing oats in the previous crop year provided a reduction in Aphanomyces in peas the following year. However, excess traffic from secondary tillage, such as harrowing and seeding, caused enough soil compaction to void any disease suppression from the oat residue. Oat green manure has also been found to suppress Aphanomyces in peas, possibly by stimulating oospores to germinate in the absence of a host plant, allowing the pathogen to be exposed to winter temperatures that would kill it, reducing spore-load in the soil.
Biological Control
Globally, some field trials have shown that arbuscular mycorrhizal fungi and certain strains of bacteria, when applied as a seed treatment, were able to improve pea seedling emergence and suppress Aphanomyces development in A. euteiches infested fields. In contrast, other studies with arbuscular mycorrhizal fungi found a positive increase in pea seedling emergence in the greenhouse but not always in the field.
Seed Treatment
INTEGO® Solo (ethaboxam), Vibrance® Maxx with INTEGO® (metalaxyl, sedaxane, fludioxonil, ethaboxam), Zeltera® Pulse (metalaxyl, inpyrfluxam, mandestrobin, ethaboxam), and Rancona Trio (carbathiin, metalaxyl, ipconazole) are registered for suppression of early season root rot caused by Aphanomyces euteiches. Tank-mixing Rancona Trio with Belmont™ 2.7 FS (peas) or INTEGO™ Solo (peas or lentil) offers control of early-season aphanomyces as per Rancona Trio product label.
These seed treatments provide approximately three weeks of early season protection after seeding, when root systems are small, actively growing, and highly susceptible to infection. There is no market-available seed treatment option that will provide protection from Aphanomyces infections that occur later in the season.
Rancona Trio is formulated as an all-in-one seed treatment. Zeltera® Pulse and Vibrance® Maxx with INTEGO® are broad-spectrum fungicidal seed treatments that are co-packed with INTEGO® Solo. Ethaboxam only has activity on the Oomycete class of water moulds (Pythium spp, Aphanomyces euteiches, Phytophthora sojae), so INTEGO® Solo is designed to be tank-mixed with a registered seed treatment that controls Fusarium and Rhizoctonia species. Also, INTEGO® Solo does not contain a colourant, so it must be coloured before treating any seed, preferably by tank-mixing with a registered seed treatment partner that will round out the disease control. Always check the compatibility of seed treatments with the intended inoculant to be used.
Genetic Resistance
Resistance to Aphanomyces root rot is quantitative, meaning that many minor genes all work together to provide partial resistance to the plant. Breeding lines of peas with partial resistance to Aphanomyces root rot do exist, however the challenge with quantitative resistance is bringing all of those minor genes into the new cross, in order to achieve stable partial resistance. In addition, some resistance sources carry undesirable node length or flower colour traits that then have to bred out of the new cross, without losing the Aphanomyces resistance. Researchers are in the process of incorporating partial resistance into adapted pea cultivars, using resistance sources from France and the United States. The Crop Development Centre at the University of Saskatchewan is also screening current lentil germplasm, as well as wild lentil species, to identify sources of Aphanomyces resistance. The use of resistant cultivars is the most effective strategy for managing Aphanomyces, but commercially competitive varieties are still a number of years away.
Summary
- Excessive soil moisture and warm soil temperatures promote Aphanomyces root rot development in peas and lentils. Wet conditions from continued rainfall will result in significant root damage and yield loss. Soil compaction can contribute to the severity of Aphanomyces root rot by reducing air-filled pores and creating the high-moisture conditions favoured by A. euteiches.
- Fusarium root rot often occurs in conjunction with Aphanomyces root rot, and can mask Aphanomyces root rot symptoms. If root rot is observed, submit a soil and/or root sample for DNA testing to confirm if Aphanomyces euteiches is present.
- A minimum six-year, and preferable eight-year, crop rotation for peas and lentils is recommended to reduce inoculum levels in the field. Control weedy host plants such as volunteer alfalfa, chickweed, and shepherd’s-purse that can serve as an alternate source of inoculum.
- Faba beans are the best pulse crop option and soybeans are also a good option for fields infested with Aphanomyces, providing they are suitable for the local growing environment. Chickpeas are considered moderately resistant and may develop low levels of infection, but could also be used as a rotational pulse crop option.
- Seed treatment will provide early-season suppression of Aphanomyces root rot in peas and lentils. INTEGO® Solo (ethaboxam) has activity on the Oomycete class of water moulds, including Aphanomyces, but needs to be tank-mixed with an approved seed treatment partner for protection from other root rot pathogens.
- Proper nutrient management can help promote the development of healthy plants and root systems to better withstand disease pressure, and adverse environmental conditions. A proven nitrogen inoculant that is compatible with the seed treatment should be used at the recommended rate. Phosphorus is an energy source in the plant, supporting root formation and nitrogen fixation. Starter phosphorus is important for early plant growth, especially under the cool soil conditions associated with early seeding.




