Research Experts: Dr. Michelle Hubbard (Agriculture and Agri-Food Canada [AAFC]), Dr. Shaun Sharpe (AAFC), and Dr. Jeff Schoenau (University of Saskatchewan [USask], Soil Science)
Introduction
Onset & Symptoms
In 2019 and 2020, a serious chickpea health issue began to emerge in commercial farm fields in southern Saskatchewan, with the epicentre around Assiniboia, Coronach, Gravelbourg and Mossbank. In both years, at the flowering to early podding stage in early to late July, reports began of chickpea plants with chlorosis (yellowing), bleaching and wilting or necrosis (death) of the top of plants, chlorosis of leaflet edges, chlorosis and/or necrosis of branches, and complete plant death in extreme cases (Figure 1). Symptoms were spread across the field in some instances and were only observed in patches in other fields. Roots of affected plants were healthy in some cases and unhealthy in others, with browning and poor root hair growth. Root nodules were non-functioning or green in some cases. Yield loss was not captured but seemed to be reduced in more seriously impacted fields, whereas fields with less severe symptoms appeared to recover.
Past Investigations & Potential Factors
Since the chickpea health issue began in 2019, researchers and agronomists have been actively working to uncover the cause and diagnose the problem. Initial investigations in 2019 found that, among the Kabuli chickpea varieties, CDC Orion appeared to be more severely affected by symptoms. An initial theory was that the breakdown of Ascochyta blight resistance in CDC Orion or the evolution of the Ascochyta rabiei fungus was causing increased disease severity. Growth chamber trials showed no difference in Ascochyta severity between the varieties, however.
Field surveys led by Dr. Sabine Banniza, Professor and Ministry of Agriculture Strategic Research Program (SRP) Chair in Pulse Crop Pathology, USask, in 2020 and 2021, found soil-borne pathogens that cause Fusarium wilt (Fusarium redolens, F. solani, F. avenaceum. F. oxysporum), and Verticillium wilt (Verticillium dahliae) present in soil samples. However, these pathogens were not consistently detected in fields exhibiting symptoms of the emerging health issue. As well, there was no difference between healthy and unhealthy chickpea fields for individual soil-borne pathogens or total pathogen load.

A 2020 field survey led by Saskatchewan Pulse Growers collected agronomic and environmental information, including chickpea variety, rainfall, tissue nutrient analysis, herbicide residue, and root or plant tissue pathogens. A total of 21 fields that exhibited the health issue symptoms were surveyed. No single consistent factor was observed between fields. However, some trends did emerge. One trend was drought followed by higher moisture input. In both years, rainfall was low in May and June, followed by precipitation events in late June/early July. In many affected fields, rainfall occurred 2-5 days before symptom emergence, and humidity conditions were high. Herbicide residue data were collected, and the residue levels for metribuzin, a common post-emergent herbicide used in chickpeas, were higher in unhealthy plants but not significantly different from healthy plants. Metribuzin residue was not found in all unhealthy samples, indicating this herbicide is, at most, only a partial explanation for the emerging health issue. Other research has identified adverse effects of metribuzin application on chickpeas, including an increase in the severity of Ascochyta blight, delayed flowering, and yield loss. Among other survey factors, nutrient analysis was inconclusive, and more testing was deemed required. As well, there was no common pathogen between unhealthy samples or a higher total pathogen load.
Another theory on the cause of the emerging chickpea health issue symptoms is that group 4 auxin herbicide carryover near the soil surface may be flushed into the soil after rainfall and then taken up by chickpea roots, causing the damage. Auxin herbicide damage from carryover produces similar symptomology to the health issue, including apically oriented wilting, chlorosis, necrosis, and growth malformations.
Microbiological investigations led by Dr. Jennifer Town, a soil microbiologist with AAFC, showed no significant differences in soil or plant bacterial, fungal, or oomycete communities between healthy and unhealthy plant samples. However, a couple of trends did emerge. DNA sequences with similarity to Fusarium hostae and Gibberella avencea were detected in healthy plant samples. Conversely, Mortierella fungus species and the Pythium oomycete species were identified in the unhealthy soil samples. An area for further microbiological investigation is the detection of plant-parasitic nematodes, which are small roundworms that live in soil and can cause crop damage.
The objective of this current research was to investigate further potential causes of the emerging chickpea health issue, including herbicide application or carryover, disease, crop variety, soil nematodes, soil nutrient status, and moisture deficit or excess, through field surveys, as well as growth chamber and greenhouse studies.
Emerging Chickpea Health Issue Research Investigations
To investigate the potential causes of the emerging chickpea health issue discussed above, a field survey and several greenhouse studies were conducted, spanning multiple agricultural disciplines. The field survey involved collecting data on environmental and agronomic factors to analyze their relationship to symptom severity. Greenhouse studies included multiple drought trials, herbicide trials, soil assays, seed lot experiments, and disease inoculation studies, as well as trials investigating the interaction of various factors, including herbicide, disease, variety, drought, nematodes, and soil fertility. Rainfall data was also modelled to analyze precipitation changes after 2019 that may contribute to the health issue.
Field Survey
Dr. Michelle Hubbard, Research Scientist, Pulse Pathology, AAFC, led a survey of commercial chickpea fields in Saskatchewan, looking at 79 fields in 2021, 81 fields in 2022, and 38 fields in 2023. The survey was carried out by Saskatchewan Pulse Growers (SPG) staff, Saskatchewan Ministry of Agriculture staff, Plant Health Officers, Saskatchewan Crop Insurance Corp, and field agronomists. Surveyors stopped at three to six sites per field and rated plants for emerging health symptom severity on a scale of 0 to 5, as follows:
- 0: no symptoms
- 1: <10% plant area affected (PAA) with wilted new growth, some leaf tip chlorosis
- 2: 10-30% PAA with chlorotic and/or necrotic tissue
- 3: 30-60% PAA chlorotic/necrotic
- 4: 60-90% PAA chlorotic/necrotic
- 5: >90% necrosis or the plant is entirely dead.
Ascochyta blight symptoms were not considered in the rating. Foliar and root tissue samples were taken at each site and underwent nutrient analysis in the laboratory of Dr. Jeff Schoenau, Professor and Ministry of Agriculture SRP Chair in Soil Nutrient Management at USask. Environmental and agronomic data collected included location, chickpea variety, seeding date and rate, crop protection products used, fertilizer rates, previous tank products, precipitation, significant weather events, and rotation history. Statistical analysis was done to understand relationships between symptom severity and agronomic and environmental factors. Dr. Mario Tenuta, Senior Industrial Research Chair in 4R Nutrient Management and Professor (Soil Ecology), University of Manitoba, classified and quantified nematodes in the soil samples taken at survey sites as well as other sites in Saskatchewan.
Forty-two rural municipalities across Saskatchewan were surveyed to assess the severity of emerging health issues over three years. The severity was lower in 2022, at 1.0, than in 2021 or 2023, which were 2.3 and 2.1, respectively (Figure 2). Severity was highest in the north-central part of the survey area in the RMs of Miry Creek, Swift Current, Coulee, and Caron (Figure 3). Notably, 2021 and 2023 were drier years than 2022, indicating that drought may increase symptom severity. However, symptom severity decreased significantly under arid conditions, but only in 2021 (Figure 4).
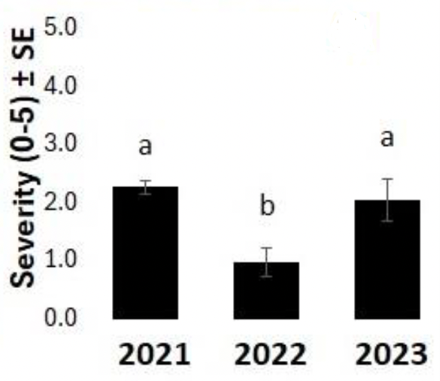

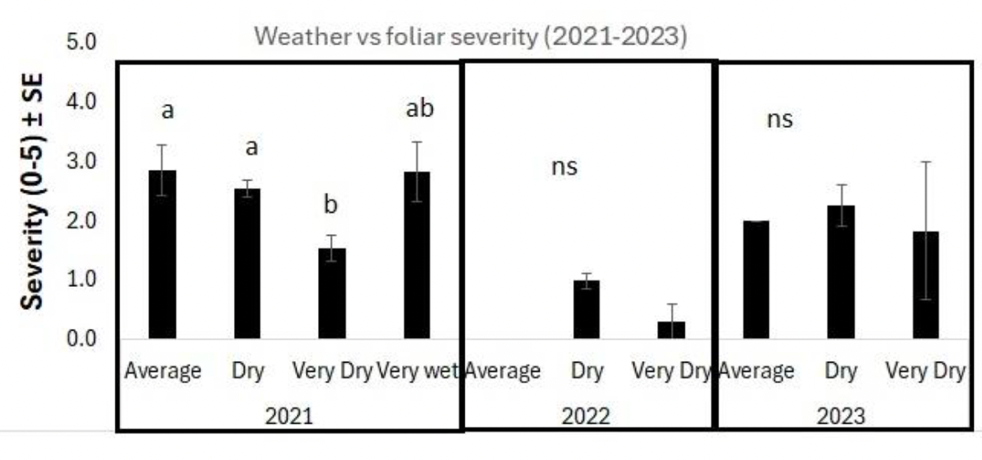
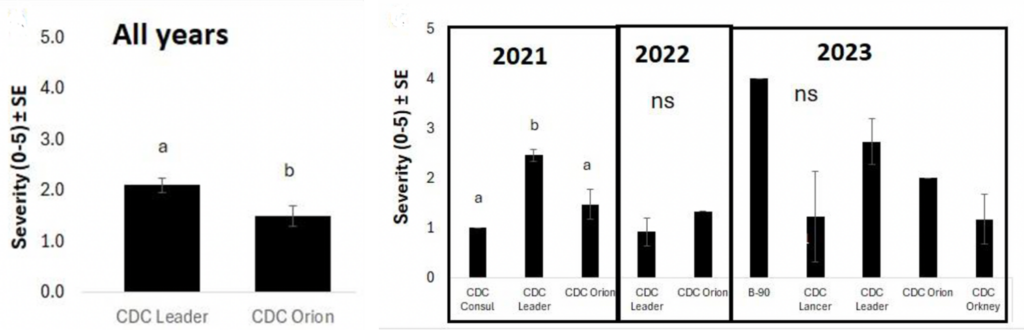
The severity of emerging health issues was not correlated with seeding rate, years between chickpea crops, inoculant type, or the application of Group 14 herbicides or glyphosate. Symptom severity was associated with chickpea variety, among other factors. Across all years, CDC Leader was at 2.1 and CDC Orion was 1.5 on a 0 to 5 scale for severity, with 5 being a dead plant. When looking at individual years, there was no difference between varieties in 2022 or 2023. In 2021, CDC Leader was rated at 2.5, whereas CDC Orion was lower at 1.5 (Figure 5). Cropping rotation also impacted symptom severity. Lentil or canola as a previous crop had more severe symptoms (rated at 2.8 and 2.7, respectively) than durum (rated at 1.7), which may be due to differences in how these crops impact soil fertility and/or nematode populations.
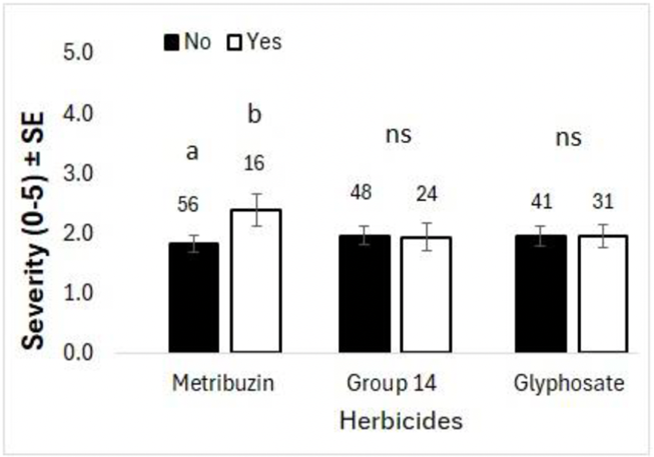
Symptom severity was correlated to metribuzin application, with ratings at 2.4 with and 1.8 without metribuzin (Figure 6). Symptoms from the emerging health issue differ from metribuzin damage, as metribuzin damage causes leaf tip chlorosis on the oldest branches. In contrast, the emerging health issue affects new growth in the top leaflets. Metribuzin damage may stress chickpeas, however, leaving them vulnerable to the causes of the emerging health issue.
The number of fungicide applications in a field and symptom severity were negatively correlated (R² of -0.35), with a severity rating of 1.0 for four fungicide applications versus ratings of 2.3 and 2.6 for one and three applications, respectively (Figure 7). More fungicide applications in a season may reduce plant stress from Ascochyta, allowing plants to better tolerate the cause of the emerging health issue. Symptom severity was significantly higher with Apron® Advance seed treatment (2.4) than Vibrance® Maxx seed treatment (0.5). However, the sample size was small, at n = 27 and n = 4, respectively. One reason could be that sedaxane, the group 7 active in Vibrance® Maxx, can reduce chickpea stress, reducing symptom development.

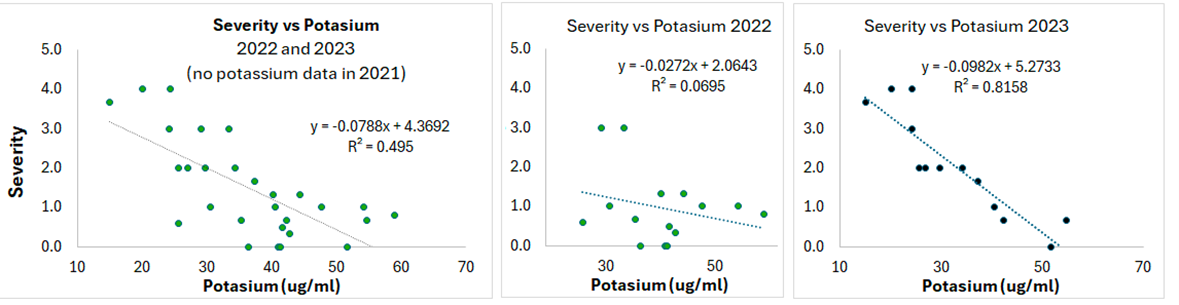
The foliar nutrient analysis showed no correlation between symptom severity and nitrogen (N), phosphorus (P), or chloride content (Cl). Lower foliar potassium (K) content was correlated to higher symptom severity, especially in 2023, with an R2 value of -0.82 (Figure 8). There was no significant difference in foliar K concentration between healthy and unhealthy samples, however. Potassium plays a role in protecting plants from pathogens by helping with the strengthening of cell walls and the synthesis of secondary metabolites. Higher foliar K may indicate better plant health and lower susceptibility to the emerging health issue. There was a positive correlation between P fertilizer rate and symptom severity (R2 coefficient of 0.12). Soil P levels were not assessed. Phosphorus is an essential nutrient for chickpeas, and yield loss from P deficiency is likely more detrimental than potential yield loss from the health issue; thus, farmers should not stop applying P, but should not overapply.
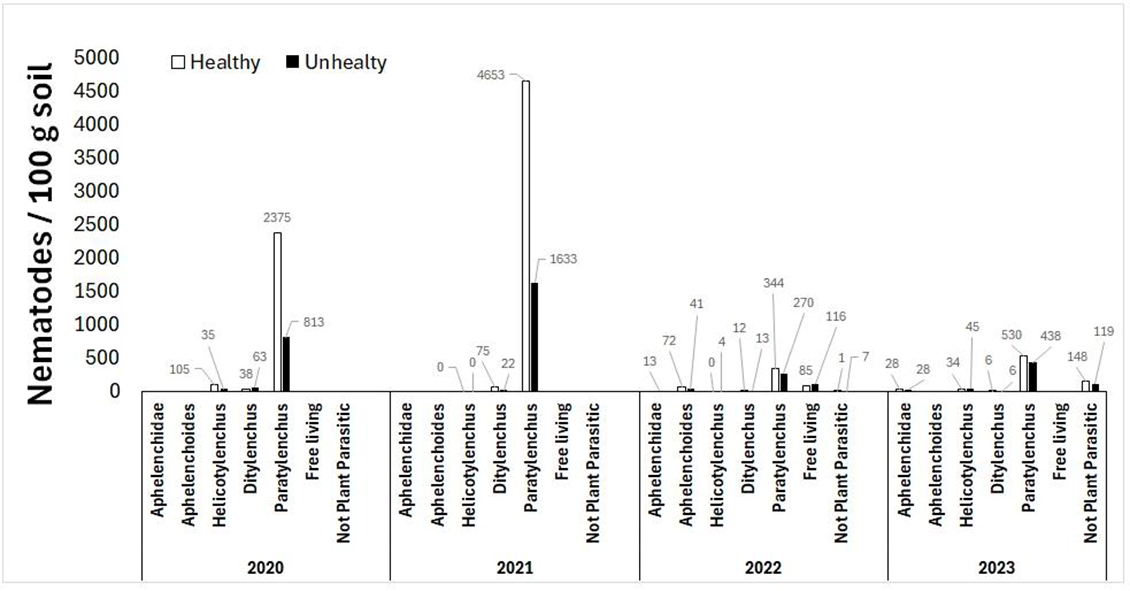
The nematode survey results showed considerable variability in nematode counts between years, species, and healthy versus unhealthy soils (Figure 9). Some trends emerged; Paratylenchus (pin) nematode counts were much higher than all other nematode species, especially in 2020 and 2021, and were more abundant in healthy than unhealthy soil in every year at 344 to 4653 nematodes per 100g of healthy soil and 270 to 1633 nematodes per 100g of unhealthy soil. Helicotylenchus (spiral) nematode counts in 2020 were 105 nematodes per 100g of soil in the healthy soil compared to 35 nematodes per 100g of soil in the unhealthy, with little difference in the rest of the survey years. Despite variability in nematode survey results, nematodes may still be a contributing factor to the emerging health issue. Nematodes feed on plant roots, causing stress to plants. Under drought stress, plants with nematode-damaged roots would not tolerate further stress well, which may contribute to the development of emerging health issue symptoms. As well, pin nematodes can reproduce on lentil or canola stubble, but not barley stubble, which may explain the impact of crop rotation on symptom severity mentioned above.
From the three-year field survey, no single agronomic or environmental factor stands out as the single cause of the emerging health issue. Instead, the results point to either a combination of stress factors that increase chickpea susceptibility to the issue or multiple factors that are the actual cause of the chickpea health issue, which may vary by field and year. Drought, the presence of nematodes, seed treatment choice, cultivar, disease, damage from metribuzin herbicide application, lower plant potassium concentration, overapplication of phosphorus fertilizer, and the interaction of these factors have emerged as potential stress factors or contributing causes of the emerging chickpea health issue.
Drought Trials
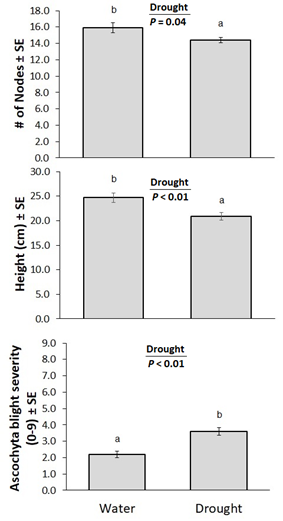
A series of drought experiments were run in the greenhouse at AAFC Swift Current between December 2021 and December 2023. The purpose of these experiments was to investigate the impact of the interaction between drought stress and crop variety on susceptibility to ascochyta blight and to determine if the combination of these two stressors could reproduce the symptoms of the emerging chickpea health issue. A total of twelve experiments were carried out, with each new one resulting from adjustments made from the previous experiment. Chickpea varieties CDC Leader, CDC Orion, CDC Orkney and CDC Pearl were used. Assessments included chlorophyll fluorescence, visual assessments, plant height, number of nodes, wilting and ascochyta blight severity. The results of the drought trials did not clarify the impact of drought on symptoms of the health issue.
The impact of drought stress on susceptibility to Ascochyta blight was also explored through five pot studies. In the first experiment, after establishment, drought stress was initiated on 10 pots of CDC Leader, which received half the amount of water, while the other 10 continued to be well watered. At the beginning of flowering, 5 of the drought-stressed and 5 of the well-watered plants were inoculated with Ascochyta rabiei. Disease severity ratings were taken 12 days after inoculation. Four other experiments were carried out comparing different chickpea varieties, including CDC Leader versus CDC Orion, and CDC Orkney versus CDC Pearl versus CDC Leader. The simulated drought conditions impacted plant performance. Drought also made plants more susceptible to Ascochyta blight in an experiment on CDC Leader only (Figure 10), perhaps because moisture stress reduced the plants’ ability to fight disease. These results were not repeated in subsequent experiments on CDC Leader and CDC Orion, or CDC Leader, CDC Orkney and CDC Pearl. The combination of drought stress and Ascochyta disease pressure impacted plant performance, but did not reproduce the symptoms of the emerging chickpea health issue, regardless of variety.
Herbicide Trials
To evaluate the interaction of herbicide and drought stress on chickpea health, greenhouse trials were carried out at AAFC’s Saskatoon Research and Development Centre (SRDC). An experiment investigating the impact of clopyralid carryover on chickpea health took place in 2021, with CDC Leader grown in either a herbicide-treated soil or a control soil. Oats, sprayed with Curtail® M at 2 L-ha (0.8 L-ac), had been grown in the herbicide-treated soil. The results showed that there was no change in chickpea leaf chlorosis or the longest branch, a measure of stunting, in the soil with previous clopyralid treatment. This experiment did not reproduce the symptoms of the emerging health issue, and clopyralid carryover is likely not a contributing factor to the emerging health issue.
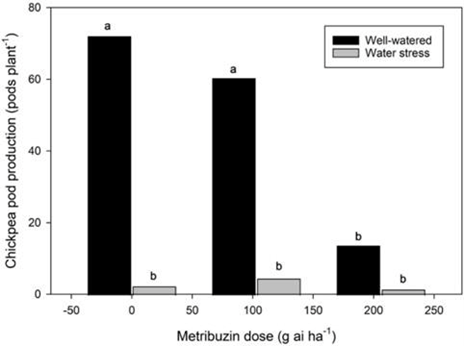
A greenhouse trial with Metribuzin was carried out in 2021 and 2022. Metribuzin was applied to chickpeas at rates of 0, 103, 206, or 413 g ai ha-1, corresponding to a control, half rate, label rate, and double rate, respectively, as recommended in the crop protection guide. Many farmers apply a lower rate or split application of metribuzin to reduce crop damage. Treatments were either well-watered or under water stress, with water restrictions beginning at the late vegetative to early flowering stages.
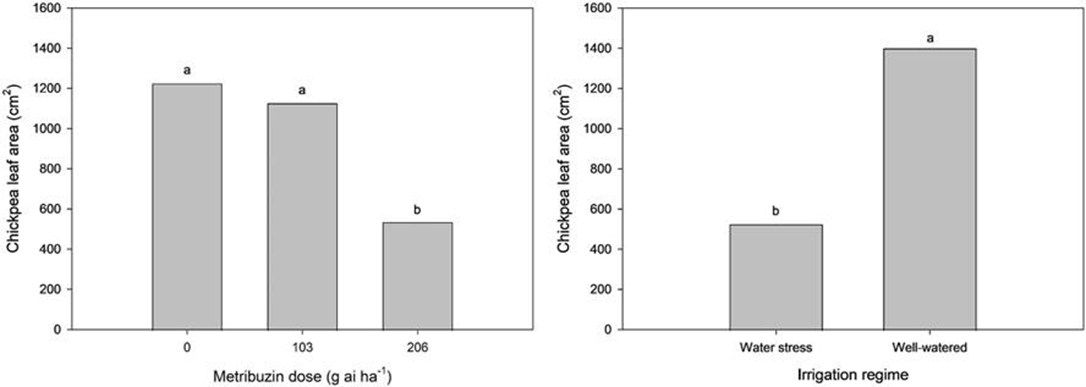
Metribuzin applied at 206 and 413 g ai ha-1 resulted in 25% and 56% mortality, respectively, at two weeks after application. At 8 weeks after application, chlorosis reached 65% for the 206 g ai ha-1 rate and 81% for the 413 g ai ha-1 rate. Metribuzin at 206 g ai ha-1 also reduced the most extended branch length by 21% at 8 weeks after treatment, reduced chickpea leaf area by over 50% (Figure 11), and reduced pod number by 75% (Figure 12), all in comparison to the half rate. Plant susceptibility to herbicides is generally higher in the greenhouse than in the field. Chickpea metribuzin injury seen in past Saskatchewan field research was 0 to 45% injury on an injury rating scale at the label rate. Farmers would likely see similar injury levels, and cutting back metribuzin rates is not recommended to prevent the development of weed resistance to metribuzin. Water stress led to 36% shorter branch length at 8 weeks after treatment, decreased chickpea leaf area by over 60%, and reduced pod production by 90%. Although drought and metribuzin application did affect chickpea health, the symptoms were not consistent with the emerging health issue symptoms, such as apical wilt, branch chlorosis and necrosis. Metribuzin application does cause plant damage and thus may be a stress factor, making plants more susceptible to the emerging chickpea health issue.
Soil Assays and Bioassays with 2020 Field Soils
To assess the impact of soil on the development of chickpea health symptoms, several greenhouse experiments were conducted. In two of the experiments, chickpea was grown in a variety of soils from chickpea-growing regions in southern Saskatchewan, as well as a control soil from the SRDC in Saskatoon. Soil was collected from two different fields in Corderre, Sask., that were infected with Fusarium species and grew a mixture of healthy and unhealthy plants. In each field, soil was collected from either hilltops or low depressions, resulting in a total of four soil samples. Another soil sample was collected from Verwood, Sask., with a high nematode count, and a sixth soil sample was from a healthy chickpea field in Maple Creek, Sask. Different irrigation regimes (adequate water, intermittent water, and drought) were also applied to each soil treatment to determine the effect of drought on chlorosis, the most extended branch length, leaf area, and pod count.
In the first of the soil comparison experiments, the hilltop and depression soil samples from one of the Fusarium-infested fields in Corderre were compared to the SRDC control. Chickpeas grown with adequate water did not exhibit chlorosis, regardless of Fusarium infestation. Plants grown in drought conditions were dead by 35 days of drought, when final measurements were taken. By the end of the experiment, chickpeas grown in Fusarium-infected soils had a 16% shorter branch length than the control soil, regardless of watering regime. As well, leaf area was on average 58% smaller in the Fusarium fields compared to the control, but only under adequate watering conditions. There was no difference in leaf area between soils when receiving intermittent watering.
In the second experiment, the four soil samples from Corderre, as well as the Verwood nematode-infested soil and the healthy soil from Maple Creek, were compared. Chickpeas grown under water stress had similar chlorosis levels at the final assessment, except for the nematode soil, which had lower chlorosis levels, like that of the thoroughly watered control. There was no difference in branch length between any soil types, regardless of irrigation scheme. All plants under the drought treatment died, so leaf area and pod production were not measured. In the treatments that received adequate water, chickpea leaf area was not affected by soil type, but pod production was. All treatments had similar pod counts except for the soil high in nematodes, which did not produce any pods. Chickpeas grown in this nematode soil also had feathery, pin-like leaves that resembled the chickpea health issue. Results from these first two soil experiments support the theory that nematodes may be a contributing factor to the emerging health issue. Contrarily, the presence of Fusarium species in the soil may impact plant growth in some situations, as was seen in the first soil experiment. Still, there was no evidence from either experiment that Fusarium species would lead to the development of chickpea health issue symptoms.
A sugar beet root length assay was conducted on the six soils from the above soil experiments. Sugar beet seeds were germinated in these soils, and root length was measured after 7 days to see if the soil inhibited root growth. Interestingly, sugar beets grown in the nematode soil had significant root growth restriction compared to the other soils. This experiment focused on sugar beet, not chickpea, and therefore, firm conclusions cannot be drawn; however, these results may indicate that high nematode counts can stress plants, potentially leading to chickpea health issues.
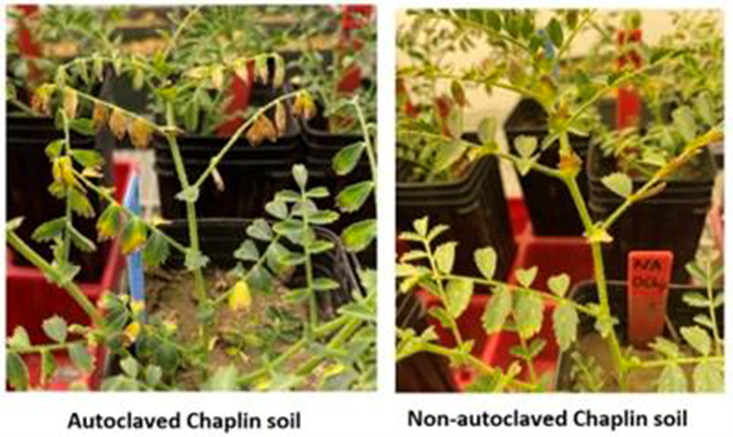
Finally, an experiment was conducted using soil collected from a field near Chaplin, Sask. When chickpea was grown in this soil indoors, symptoms similar to those of the emerging health issue developed. The soil was sent to AAFC Swift Current, where half the soil was autoclaved to kill all soil microorganisms, including soilborne pathogens. Chickpea was grown in each soil and assessed for emerging health issue symptom severity using the field survey 0-5 scale. If health issue symptoms are the result of soilborne pathogens or other soil biological factors, no symptoms should develop in the autoclaved soil. Surprisingly, emerging health issue symptoms appeared first and more severely on chickpeas grown in the autoclaved soil (Figure 13). The emerging health issue symptoms that developed in the autoclaved soil may have resulted from plant stress due to a lack of beneficial soil microbes to help chickpea growth, or structural changes caused by soil autoclaving.
Seed Lot Experiments
To determine if the emerging chickpea health issue is seedborne, three seed lots of CDC Leader, two planted in 2022 and one produced in 2022, were grown in autoclaved soil in the greenhouse. The first seed lot was treated with Apron® Advance and 20g Cruiser®, the second seed lot was produced by chickpea grown from the first seed lot in the field and was untreated, and the third was treated with only Apron® Advance. A second experiment also included a control seed lot of CDC Leader from which chickpea with symptoms of the emerging health issue had not developed when used in other experiments. Symptoms of the emerging health issue appeared in all three seed lots compared in the pot study, as well as in the control seed lot, leading to the conclusion that the health issue is not seedborne.
Metribuzin, Ascochyta blight, and Chickpea Variety Interaction
To determine if the interaction of metribuzin application, Ascochyta inoculation, and chickpea variety can induce symptoms consistent with the emerging health issue, a growth chamber pot study was conducted at the SRDC. Metribuzin was applied at rates of 0, 103, and 206 g ai ha-1. A portion of pots of CDC Orion and CDC Leader were inoculated with Ascochyta, while the rest were left uninoculated. The experiment was carried out twice. Data collected included plant height, herbicide injury, chickpea wilted segments, chickpea wilt severity, and disease ratings.

Most plants did not show symptoms reflective of the emerging health issue, except for one pot of CDC Orion in experiment two that was inoculated with ascochyta and did not receive a metribuzin application (Figure 14). Results were inconsistent between the experiments. Overall, the metribuzin rate impacted the parameters measured more in experiment one. This is likely because plants were larger in experiment one than in experiment two when they received a metribuzin application and thus sustained more injury. Herbicide injury at the 206 g ai ha-1 rate was 91% in experiment one versus 32% injury in experiment 2. Ascochyta severity significantly increased with metribuzin dose in experiment one from 2 to 4 to 8 for 0, 103, and 206 g ai ha-1 metribuzin, respectively. In experiment two, Ascochyta severity significantly increased from a severity rating of 1 to 6 with uninoculated versus inoculated. Chickpea vegetative biomass was reduced with metribuzin application in both experiments, by 27% in the first and 37% in the second experiment for the 103 g ai ha-1 rate compared to no metribuzin application. Health issue symptoms did not develop with the interaction of metribuzin application and Ascochyta blight inoculation, suggesting that the combination of these two factors is not the cause of the emerging health issue.
Drought, Nematodes, Microbiome & Nutrient Status Interaction
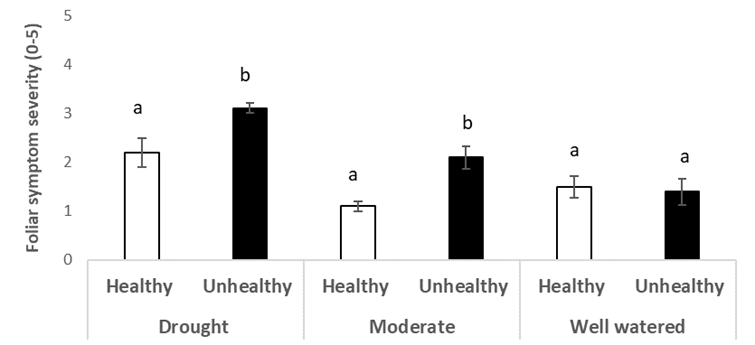
In 2023, the regional chickpea variety trial in Redvers, Sask. exhibited symptoms of the emerging health issue in specific plots in the field (Figure 15). Soil samples were taken from healthy (H) and unhealthy (UH) areas immediately after harvest. CDC Pearl was grown in the greenhouse under three levels of drought conditions: well-watered, moderate drought, and severe drought.

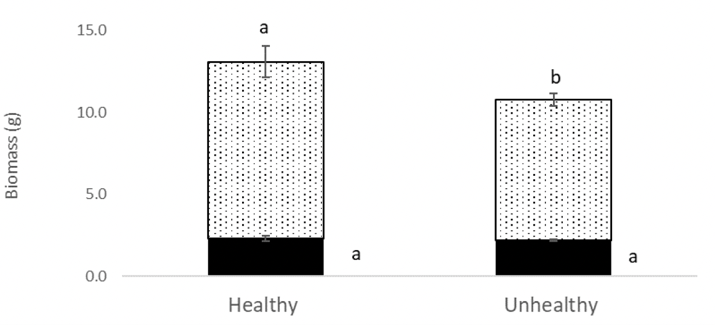
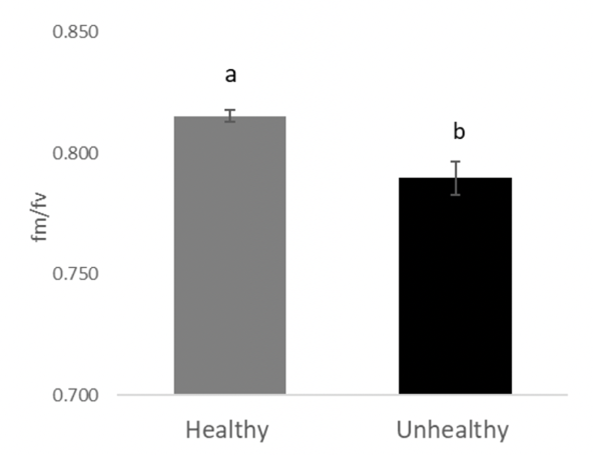
The chickpea health issue symptoms did not develop in either the H or UH soil. Compared to the H soil, plants grown in the UH soil had lower above-ground plant biomass (Figure 16) and chlorophyll fluorescence (Figure 17), as well as more severe foliar symptoms in the severe and moderate drought conditions only (Figure 18). There was no difference between the healthy and unhealthy soil in plant height, node or pod counts. The H soil was significantly higher in potassium (K) than the UH soil at 338.9 ppm and 174.4 ppm respectively, and there were small but statistically significant differences in sulphates (SO4–), nitrates (NO3–), phosphate (PO4–), and electrical conductivity (EC)(Table 1). Counts for pin (Paratylenchus) nematodes did not differ between the H and UH soil types and were very low compared to the survey of soil nematode counts earlier in this report. There were significantly higher counts of spiral (Helicotylenchus) nematodes in the UH soil, at close to 400 per 100g of dry soil, compared to less than 50 in the H soil. In the soil nematode survey discussed above, spiral nematode levels were higher at healthy sites than at unhealthy sites in 1 of 3 survey years. Spiral nematodes may have contributed to the emerging health symptoms at Redvers, but do not always seem to be the cause of symptoms based on the survey results.
The soil microbiome analysis, which measured differences in bacterial, fungal, and oomycete soil community composition, showed significant differences between the H and UH soil, with no significant differences in community composition between drought levels. Metabolites from chickpea leaves and roots were significantly different between H and UH soils, as well as between drought treatments; drought had a greater impact on metabolites than the soil type. Further work is underway to identify metabolites and link them to the causes of the symptoms. This work is likely to provide important insight into the effect of drought and high spiral nematode counts on chickpea.
Table 1: Nutrients, total and organic carbon, pH, and EC measured in healthy (H) and unhealthy (UH) soil. Statistically significant differences within a nutrient are shown by different letters (p<0.05)
| Soil Condition | K | SO4– | NO3– | PO4– | Total-C | Org-C | pH | EC |
|---|---|---|---|---|---|---|---|---|
| Healthy (H) | 338.9 a | 8.3 a | 28.6 a | 15.1 a | 2.9 a | 2.6 a | 7.6 a | 1.1 a |
| Unhealthy (UH) | 174.4 b | 4.9 b | 33.2 b | 21.1 b | 2.9 a | 2.05 a | 7.7 a | 0.96 b |
Modeling
Rainfall data for Assiniboia from Environment Canada was gathered to create a standard model for rainfall averages for 2015 and 2016, which were considered the control years with healthy chickpea yields. The years 2019 to 2021, when the emerging chickpea health issue began, were then compared to the model to see if rainfall deviated from the norm. Rainfall was variable between the three years for May and June, but was abnormally low in July for all three years. The model aligns with what was observed in the field, as the onset of chickpea emerging health issue symptoms usually occurred in July after rainfall events, following drought stress earlier in the growing season.
Conclusions & Recommendations
The causes of the chickpea emerging health issue remain unknown; however, some potential contributing factors have emerged from this work. Possible factors include the presence of soil nematodes, especially pin and spiral nematodes, low levels of potassium, imbalance in the soil microbial community, drought, Ascochyta disease pressure, or metribuzin application. Results indicated that factors that contribute to plant stress, such as nematode root feeding, disease pressure, drought, or metribuzin damage, may reduce a chickpea plant’s ability to withstand the causes of the emerging health issue symptoms. Some management factors that farmers could employ to reduce stress on their chickpea crop would be ensuring adequate soil K through soil testing and fertilizer application, avoiding over-applying phosphorus fertilizer, fungicide application, growing chickpea after durum rather than lentil or canola, and the use of a seed treatment with the fungicide active ingredient sedaxane. No chickpea variety proved to be consistently less susceptible to the health issue. Thus, farmers should choose the variety that performs best in their region.
Farmers should keep in mind that, in general, symptoms from the emerging chickpea health issue are usually mild. Plants often recover, and while no yield data has been collected, yield loss does not appear to be significant in most affected fields. Therefore, farmers should not remove chickpeas from the rotation; the benefits of growing this crop, especially its high value and resistance to Aphanomyces root rot, far outweigh any potential loss from the emerging chickpea health issue.