By Jennifer Bogdan, PAg, CCA
Grasshoppers can become a problem in pulse crops in years of hot, dry weather. Their populations can build up to extreme numbers resulting in excessive defoliation, and pod and seed damage to pulses. Lentils are especially sensitive to some grasshopper species as pod-clipping by these insects can cause direct and significant yield losses. Weather and natural enemies can greatly impact the size of the grasshopper population for the following year.
Grasshopper Pest Species in Saskatchewan Pulse Crops

Source: Jennifer Bogdan
Of the over 80 species of grasshoppers on the Prairies, only four are typically considered crop pest species in Saskatchewan, and just three of these species are a significant threat to pulse crops. “Pest” species are characterized by (1) their broad range of host plants that they feed on, and (2) large numbers leading to economic damage to crops.
The three grasshopper species of interest for pulse crops are the two- striped grasshopper (Melanoplus bivittatus), Packard’s grasshopper (Melanoplus packardii), and the migratory grasshopper (also referred to as the lesser migratory grasshopper, Melanoplus sanguinipes). These species all belong in the same subfamily of spur-throated grasshoppers (Melanoplinae). “Spur-throated” refers to the short spine or spur present between the front legs of these species, in the ‘throat’ area (Figure 1). If it has a spur-throat, it is likely a pulse pest species.
Note that the clear-winged grasshopper (Camnula pellucida), the fourth main pest species in Saskatchewan crops, feeds primarily on grasses and cereal crops, but not on pulse crops. It is part of a different subfamily, the band-winged grasshoppers (Oedipodinae) which typically have red, yellow, or black hind wings visible during flight, and make a clacking sound when they fly. The clear-winged grasshopper is an exception to this group due to its clear, not coloured, wings. Clear- winged grasshoppers have conspicuous dark bands on the forewings, and do not have a spur-throat, distinguishing it from the species that feed on pulse crops.
Native Grasshoppers
Not every grasshopper is a pest species. A grasshopper with one or more of the following characteristics is a native species:
- Flying before June – meaning they are adults at this time (the pest species overwinter as eggs).
- Colourful wings – red, orange, yellow, or black hind wings that can be seen during flight.
- Noisy – singing or clacking sounds while on the ground or in-flight (the pest species are silent).
Grasshopper Identification
Photos of grasshopper species can be found in Appendix 1.
Two-striped Grasshopper
The two-striped grasshopper is the largest of three pulse crop pest species, with males measuring 24 to 28 mm (approximately 1 inch) long and females up to 40 mm (1.6 inches) long. Adults are yellowish- green with two pale yellow stripes running from the eyes to the tip of the forewings. The hind femur also has a distinct black stripe extending down the length of it. Nymphs can be green, yellow-orange, tan, or brown, but still have the black stripe on the hind femur as well as two black stripes on the thorax.
Two-striped grasshoppers feed on both grasses and broadleaf plants, such as pulses, and require broadleaves as part of their diet to achieve maximum growth. They will feed on cereals but these crops are not their preferred food. Two-striped grasshoppers prefer lush vegetation such as that found near water bodies and in ditches. For this reason, they are also prevalent in areas with heavier textured (clay) soils as more soil moisture is available for plant growth. Egg-laying occurs in these heavier textured soils along roadsides, ditches, and fences.
Packard’s Grasshopper
Adults are dark yellow to greyish in colour and are 27 to 32 mm (1.1 to 1.3 inches) long. Like the two-striped grasshopper, Packard’s grasshoppers have two pale yellow stripes extending from the eyes and down the thorax. However, in Packard’s grasshoppers, the stripes stop at the end of the thorax, whereas the stripes of the two-striped grasshopper continue from the thorax down to the very end of the forewings. Packard’s grasshoppers also typically have a blue tibia on the hind legs. Nymphs are green (sometimes lime-green) and have black, pepper-like dots scattered on their bodies and hind leg femurs.
Packard’s grasshoppers feed on both grasses and broadleaf plants, but they do prefer legumes (pulses and alfalfa). Unlike the two-striped grasshopper, Packard’s grasshoppers prefer sandier soils and open areas with drier conditions to inhabit. They are a less common species than two-striped grasshoppers.
Migratory Grasshopper
Adult migratory grasshoppers are the smallest of the pulse pest species, with males reaching about 20 mm (0.8 inches) and females about 28 mm (1.1 inches) long. Migratory grasshoppers are dark greyish-brown with rows of small dark spots on the forewings, giving them a mottled appearance. They have black bands on the femur of their hind legs and the hind tibia is typically red but can also vary to yellow or blue. Behind their eye is a stripe that runs along the side of their head and onto their thorax, making it look like they are wearing a mask. In nymphs, these black bands appear on the top of the thorax but are on the side of the thorax at the adult stage. Nymphs look like adults in their mottled black appearance. Migratory grasshoppers can be confused for the closely related Bruner’s spur-throated grasshopper (Melanoplus bruneri), which is slightly larger and darker, and less common than the migratory grasshopper, although this species has occasionally seen outbreaks in localized areas of Saskatchewan and Alberta.
Migratory grasshoppers are omnivores and have a very mixed diet. They prefer to feed on broadleaves, although will readily eat grasses and cereals, consuming all stages of the plant (leaves, stems, flowers, fruits, seeds). They will scavenge on ground litter, dead insects, and dried manure, and during outbreaks when food is scarce, they will even feed on trees, weaker grasshopper individuals, and cotton or wool fabrics. Some grasshopper species, including migratory, can clip the stem just below the seed pod, contributing to heavy yield reductions.
Under high population densities, there is a greater need to search for more food and the behaviour of migratory grasshoppers changes. They become more gregarious and begin migrating in large groups, as their name suggests. Bands of older nymphs (3rd to 6th instars) can move 8 to 16 km (5 to 10 miles) from their hatching site in search of the nearest available crop. Adults are strong fliers, flying at speeds of 16 to 19 km (10 to 12 miles) per hour, even faster when carried by the wind, and moving 48 km (30 miles) or more per day.
Life Cycle

Source: Jennifer Bogdan
The life cycles of pest grasshopper species are similar. Female grasshoppers lay their eggs in the soil by boring a hole with their abdomen about 2 to 5 cm (1 to 2 inches) deep. The eggs are approximately 0.5 cm long and creamy yellow-brown. A foamy secretion is deposited onto the egg cluster and hardens into an egg pods which offers protection for the eggs (Figure 2). The number of eggs per pod can vary between grasshopper species, but 30 to 60 eggs per pod is typical for the pest species.
Egg development begins as soon as the eggs are laid. As soil temperatures cool below 10ºC, embryo development pauses for the winter and resumes as soil temperatures increase the following spring. All pest grasshopper species on the Prairies overwinter as eggs. Hatching occurs over a minimum two-week period, typically beginning in late May.
The nymphs pass through five growth stages (instars), feeding and moulting for 4 to 6 weeks until they reach the adult stage in approximately mid-to-late July. Migratory grasshoppers occasionally have a sixth instar. Newly-hatched nymphs are approximately 3 mm (1/8 of an inch) long. Upon hatching, the nymphs begin feeding on whatever green vegetation is available to them since they cannot travel far at this stage. The presence of large numbers of third instar nymphs is an indication that warm weather has helped facilitate a successful hatch and grasshopper development is rapidly occurring. On the Prairies, the third instar appears around mid-June to early July, depending on species, heat accumulation, and latitude. By the fourth instar, feeding activity and subsequent crop damage can become significant.
Once the adult stage is reached, the grasshopper has wings and is reproductively mature. Mating begins about two weeks after this final moult, followed by egg-laying within 1 to 2 weeks. Females lay eggs from late July into the fall, until conditions become unfavourable (generally the first hard frost). Typical egg-laying sites include ditches, roadsides, fence lines, shelterbelts, weedy areas, as well as alfalfa fields, pasture, and some fields having lush growth (late-seeded crops and/or weedy fields). There is only one generation per year of pest grasshoppers on the Prairies.
Effect of Environment on Grasshopper Populations
Temperature
Grasshopper populations are affected by temperature and moisture. The size of this year’s spring grasshopper population is heavily influenced by the effect of temperature on last year’s grasshoppers. Warm temperatures increase the rate of development through the insects’ growth stages, allowing them to reach adulthood faster. This gives the females the opportunity to lay more eggs, leading to a higher base population of grasshoppers the following year. A warm, open fall continues to provide green plant growth for food and extends the egg-laying period for the females. In addition, favourable conditions after the eggs are laid allow the embryos to reach a greater developmental stage before pausing for the winter. When temperatures rise the following spring, these embryos will already be at a more advanced stage leading to an earlier hatch. Crops at this time will be younger and less tolerant to defoliation.
Observations of migratory grasshoppers in Northern Montana in 1924 identified the impact of daily temperature changes on feeding behaviour. The majority of the feeding was done in the morning when temperatures were between 18 and 29ºC, optimally between 21 and 27ºC. Little to no feeding occurred below 15ºC or above 32ºC, or during cold, cloudy, rainy, or moderately to highly windy weather. Due to their fat reserves, many grasshopper species can survive several days without feeding during unfavourable weather and will feed heavily once conditions improve.
Cold winter temperatures alone have little impact on grasshopper eggs, although there is evidence of reduced grasshopper populations after experiencing severe cold without snow cover. Despite the bitter air temperatures experienced during a Prairie winter, the soil temperature does not cool to the same degree and rarely falls under -10ºC in the field. Research from Agriculture and Agri-Food Canada in Lethbridge has found that grasshopper eggs can survive temperatures down to -15ºC at 5 cm (2 inches) deep with little mortality. Snow cover will also provide insulation for the eggs, keeping in mind that the eggs are conveniently laid in places that tend to accumulate snow. Winter wheat can be used as a guideline for estimating egg survival – if the winter wheat has successfully overwintered, so have the grasshopper eggs.
Cool spring temperatures have little effect on the mortality of young nymphs. Early instar nymphs are quite hardy and can even survive freezing temperatures for short periods of time. The main effects of a cool spring are slower insect development and crop growth.
Moisture
The amount of precipitation (rain and snow) also plays a role in grasshopper populations. There are two critical times when rainfall can have a significant effect on the population: in the fall during egg- laying and in the spring during egg-hatch. Rain in the fall makes for poor egg-laying conditions for the females; grasshoppers are more active on warm, sunny days compared to cloudy, rainy days. A wet fall translates into fewer eggs laid. Rain in the spring makes it harder for the newly hatched nymphs to survive. A heavy downpour during hatching can drown new nymphs. Cloudy weather also slows the development of grasshoppers. Although cool, wet weather in June will not significantly affect grasshopper populations, the humid conditions combined with the heat of July can promote fungal infections in grasshoppers.
It is important to remember that the damage caused by grasshoppers is related to the growth of the crop, which is also influenced by temperature and precipitation. Good moisture during the spring and throughout the growing season will promote lush crop with lots of leafy growth. These dense crop canopies can tolerate a lot more feeding from grasshoppers, even under higher insect pressure. Conversely, crops under dry conditions are thin, without a lot of vegetative material. In these situations, even a small grasshopper population can do a lot of damage. Table 1 summarizes the impact of above and below average temperatures and precipitation on grasshopper populations during the growing season.
Table 1: Effect of Growing Season Temperature and Precipitation on Grasshopper Populations
| Temperature | Precipitation | |
| Dry | Wet | |
| Warm | Early egg hatch and rapid development of nymphs. Adult stage is reached sooner. Egg-laying gets an early start; females able to lay more eggs. Increased embryo development can occur before winter, so embryos are in a more advanced stage the following spring, leading to an earlier hatch. Hot, dry conditions also impede crop growth, decreasing their tolerance to feeding. | Fungal diseases increase in grasshopper populations, providing control. Crops become lush and can tolerate more feeding. |
| Cool | Egg development is slower, delaying the hatch. Nymph progression through the instar stages takes longer. Adult females start laying eggs later and will not lay as many eggs. | Hatch and nymph development delayed. Egg-laying window for female adults is reduced and fewer eggs laid, reducing populations the following spring. Heavier crop growth will increase tolerance to feeding. |
Impact of Grasshoppers on Pulse Crops
Peas
Peas are not a preferred host plant for grasshoppers, but grasshoppers will feed on a pea crop in the absence of other food sources. Most feeding damage occurs at the field edges where grasshoppers have moved into the crop from ditches and roadways. Typically, counts of 10 grasshoppers/m2 and less will not cause economic losses in peas.
Lentils
Grasshoppers do not care to feed on the foliage of lentils, and the dense canopy of a lentil crop creates cool, moist conditions that grasshoppers tend to avoid (they prefer warm, open areas). However, grasshoppers will inhabit the upper canopy structures, feeding on flower buds, opened flowers, and developing pods. The loss of these structures not only affects yield, but also triggers the plant to produce more flowers resulting in delayed maturity of an already indeterminate plant species. Damaged pods are also more prone to disease, shattering loss, seed loss, and seed staining. Grasshoppers can also clip the pods from the plant causing direct yield loss. Because of the specific damage grasshoppers cause to lentils, the economic threshold is lowest for this most sensitive pulse crop species (2 grasshoppers/m2).
Chickpeas
Chickpea leaves, stems, and pods are covered in hair-like glands (trichomes) that secrete malic and oxalic acids which deter insect feeding. Grasshoppers typically do not cause damage in chickpeas, unless food supplies are low. More often, the grasshoppers will be feeding on weeds within the chickpea crop rather than on the chickpeas themselves. If chickpea feeding does occur, it is usually at the seedling stage, particularly along the field margins along ditches and roadsides, and/or in years of high grasshopper populations.
Faba Beans
Faba beans are favoured by grasshoppers and these insects will selectively choose to feed on faba bean even when other plant choices are available. Grasshoppers will defoliate faba bean plants during the vegetative stage, then damage flower buds and pods during the reproductive stage of the plant. Pod and seed formation are affected by grasshopper feeding, reducing yield. Economic thresholds have not been established for grasshoppers in faba beans.
Soybeans
Soybeans are not a preferred host plant for grasshoppers, although under heavy infestations, feeding may occur especially along the field margins as the grasshoppers move into the field. Soybeans are quite tolerant to foliar feeding. Grasshoppers in soybean fields may be feeding on weeds rather than on the soybean plants.
Dry Beans
Dry beans are susceptible to feeding by grasshoppers, with damage often concentrated on the field margins where the grasshoppers move into the crop. Grasshoppers defoliate dry bean plants, affecting yield. The indeterminate growth of some types of dry beans makes them an attractive crop for grasshoppers to move into later in the season when other crops have started to mature and become less palatable.
Fenugreek
Fenugreek can experience economic losses under heavy infestations of grasshoppers feeding on mature seed pods.
Grain Quality
The tolerance for insect parts in pulse crops is 0.02% – a very small amount. At harvest, whole grasshopper bodies can show up in seed samples. Over time, the bodies desiccate in storage as well as during drying or aeration. The bodies break apart naturally in the bin and disintegrate further under handling of the product. In the end, most of the insect parts are cleaned out in the dockage, but grasshopper heads can remain in the clean seed as they are the same diameter as a pea or a large green lentil. Because the heads are only a small portion of the grasshoppers originally present in the sample, they are usually not in high enough concentration to cause downgrading of the sample due to insect parts, although there have been some instances where downgrading has occurred under high grasshopper infestations (Figure 3).
Seeds harvested from heavily infested fields can also have staining and odour. Seed staining due to insects is considered “earth-tag” and graded accordingly. Grain inspectors also check these samples for unnatural odour. Downgrading due to seed staining and odour from grasshoppers is not very common, but is possible under high infestations.

Source: Viterra Quality Control
Monitoring for Grasshoppers
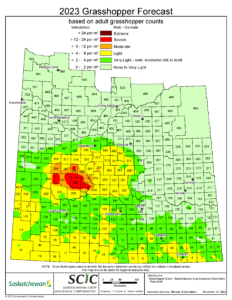
Source: Saskatchewan Ministry of Agriculture
Forecast Map
Each year, the Saskatchewan Ministry of Agriculture coordinates and Saskatchewan Crop Insurance Corporation conducts a grasshopper survey, sampling approximately 1200 roadside sites from early August until early September. Adult grasshoppers per m2 are recorded; the numbers of these egg-laying grasshoppers are used to estimate the size of the overwintering egg population that will hatch into grasshoppers the next spring. The data is compiled to produce a forecast map for the following year (Figure 4). This map is a useful tool to help predict grasshopper population levels across larger areas of the province. Because smaller, localized hotspots are smoothed out during the production of this map, it should serve as a general guideline and does not replace scouting of individual fields, especially in areas that saw grasshopper outbreaks the previous year.
Predicted Development Modelling
Agriculture and Agri-Food Canada produces weekly in-season maps and graphs to predict the development of grasshopper eggs and nymphal stages, using growing degree day accumulation and other environmental factors (Figure 5). The model is based on the biological parameters of the migratory grasshopper. These weekly updates can be accessed by subscribing to the Prairie Pest Monitoring Network at prairiepest.ca.
In-season Scouting
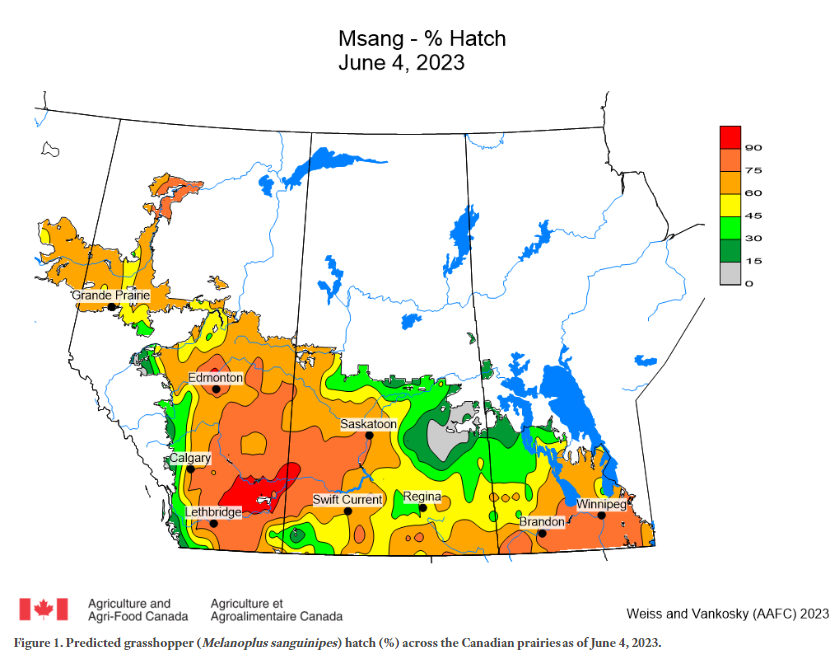
Source: Weiss and Vankosky, Agriculture and Agri-Food Canada, 2022
There are two main timings to scout for grasshoppers, especially for sensitive pulse crops such as lentils: (1) earlier in the season at egg- laying sites after the hatch begins, focusing on the nymphal growth stage; and, (2) mid-season in the field focusing on crop stage and damage.
Scouting for grasshoppers should begin in late May when the hatch begins. Look for small grasshopper nymphs in areas where egg-laying occurred the previous fall, such as field edges, ditches, fence lines, and roadsides. Early-season scouting can help gain an understanding of the size of the hatch and potential grasshopper population, as well as when the hatch begun. Ideally, these hatch sites should be checked weekly to monitor the nymph growth stage, estimate population size (number of grasshoppers per m2), and track movement of the grasshoppers into sensitive fields.
Once grasshoppers have moved into the crop, remain vigilant with scouting to monitor the situation. Take note of the following:
- Crop damage relative to the growth and development of the crop – what growth stage is the crop at? How well did the crop establish? Is the canopy thick and dense? What type of feeding damage are the grasshoppers doing (vegetative vs reproductive structures)? Are they feeding on the crop itself or just weeds within the crop?
- Changes in grasshopper population size – has the population size increased or decreased relative to the population estimated at the hatch sites? Is the population near economic threshold levels?
- Changes in grasshopper growth stage – how quickly have the nymphs been moving through their instar stages? Are they getting close to the third and fourth instar stages? Are they approaching the adult stage?
- Growing conditions – are the growing conditions favourable to crop growth for it to tolerate some feeding damage? Are the growing conditions favouring or hindering grasshopper development and activity?
- Grasshopper location – are the grasshoppers concentrated near the field margins or are they spreading further toward the centre of the field? How far into the field have they moved? If threshold numbers have been reached, would spraying just the margins be sufficient for control?
Counting grasshoppers is a challenge because they move away when disturbed, so an estimate of their numbers is the best that can be done. Accurate counts are even more difficult under high grasshopper populations. To count grasshoppers per m2, visualize a square metre approximately five paces in front of you. Slowly approach the square metre, counting any grasshoppers that jump outside the boundaries of the square. It may be helpful to walk in a zigzag towards the square rather than straight on, so you don’t force grasshoppers into the square while they are trying to move away from you. Once you reach the square, lightly brush the plants to count the remaining grasshoppers in the square. Repeat this process for a total of at least 5 sites.
Vulnerable Situations for Pulse Crops
Some situations can cause a migration of grasshoppers from one area into another, with food being the usual driving factor. For example, a hay field that is cut could force grasshoppers to move into a neighbouring field if their food supply has suddenly become more scarce. Grasshoppers will search for ‘greener pastures’, especially when their populations are high, and pulse crops can be an attractive crop to move into, particularly those with a later maturity that stay greener longer into the season. Pay attention to the areas surrounding pulse crop fields to identify any potential situations that could encourage grasshopper movement into a sensitive pulse crop.
Management Considerations
Chemical Control
Grasshopper Stage
From an insect growth stage perspective, the optimal time to control grasshoppers is when the majority of the population has reached the 3rd instar stage. This stage typically occurs in mid-June to early July, but is dependent on heat accumulation. The end of the optimal window for control is at the 4th instar. Beginning insecticide applications at the 3rd instar provides some leeway in case spraying is delayed (poor weather, booking custom applicator, product availability, etc). By the 3rd instar, most of the eggs have hatched so the application will cover as many nymphs as possible. Spraying before the 3rd instar means that a certain amount of the population still has not hatched, and insecticides will not control eggs since they are underground.
Later instars and adults are more difficult to control. Individual insecticide labels will specify if grasshoppers need to be sprayed at earlier stages and if the product will control adults. The stage of the grasshoppers also determines the rate of insecticide needed – larger grasshoppers require higher rates. Higher rates mean more product used which translates to increased cost of control.
Grasshopper Staging Considerations for Control
Paying attention to the nymph instar stage can help to decide when to implement control measures. If there is a heavy population of nymphs in the crop, consider the following guidelines based on the majority instar stage present:
- 1st instars – these nymphs are at a young age and there will still be a lot of eggs left to hatch. An insecticide application at this stage would not control any unhatched nymphs.
- 2nd instars – nymphs are mobile enough to move into crops or adjacent fields if they have used up their food supply. There will still be eggs left to hatch at this stage. Unless the crop is being devastated by large numbers of nymphs, it is better to wait until more grasshoppers have hatched before spraying.
- 3rd instars – once most of the nymphs have reached the 3rd instar, the vast majority of the eggs will have hatched. This is the beginning of the optimal control window.
- 4th instars – maximum feeding is beginning at this stage. This is the end of the optimal control window.
- 5th (& 6th) instars – the final nymphal instar stage. Last chance to control nymphs before they become adults (the most difficult stage to control). High insecticide rates are required, not all insecticide products may be effective on large nymphs, and optimal control may not be achieved.
- Adults – growth and reproductive development is complete. Wings allow the grasshoppers to fly further into the crop or spread to other fields. Females begin to lay eggs which have the potential to impact the next year’s crop. High insecticide rates are required, not all insecticide products may be effective, and expectations for control may be reduced depending on product being used.
Economic Thresholds
For a sensitive crop like lentils, when nymphs have reached the 3rd instar the assessments in Table 2 may be considered. These guidelines are generalized and depend on the individual situation such as the value of the crop being scouted or the crop bordering the area being scouted where grasshoppers could potentially move into, the crop growth stage, and the overall health of the crop, including plant density and stressors (drought, etc).
Table 2: General Grasshopper Control Guidelines
| Control | Field Grasshoppers/m2 | Roadside Grasshoppers/m2 |
|---|---|---|
| Control not usually required | 0-6 | 13-24 |
| Control may be required | 0-12 | 13+ |
| Control usually required | 7-12 | 25+ |
By the time the lentils have reached flowering and podding, the grasshoppers may be at a larger growth stage (past the 3rd and 4th instar). Therefore, using the general guidelines presented in Table 2 may help to manage potential hot spots that could become a challenge in a lentil crop. However, since grasshopper populations can be impacted by external factors (weather, disease, etc) between the 3rd instar and when the lentils are susceptible (flowering and podding), remaining focused on actual crop damage, economic thresholds, and crop stage allows for a more measured approach when possible.
Source: Jason McMechan, University of Nebraska-Lincoln
For lentils, the economic threshold is 2 grasshoppers per m2 during flowering and podding stages. Because the dense canopy of lentils at this time creates an unfavourable micro-climate for grasshoppers, the grasshoppers will likely be concentrated at the field margins. Scout the whole field to get an idea of where the most damage is. In some cases, only the field margins and areas where the grasshoppers are moving in from (roadsides, ditches, fence lines) require treatment.
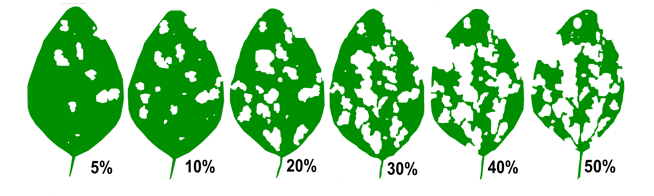
While there is no established economic threshold for soybeans or dry beans, a nominal threshold based on known information about defoliation can be used. Soybeans can generally withstand quite a bit of defoliation before yield is impacted. Select two random plants in at least five sites to assess leaves for defoliation. For grasshoppers, the nominal threshold used for soybeans and dry beans is over 30% defoliation at vegetative stages (pre-flowering), 15% defoliation from flowering to pod-fill, or 25% defoliation from pod-fill to maturity (Figure 6). If grasshoppers are feeding directly on the pods, control should be taken sooner.
Grasshopper Location
When they are younger, grasshoppers tend to be concentrated in the field margins where they have migrated from ditches and roadsides. Spraying the perimeter of the field can be very effective in providing control if crop damage is occurring and populations are high. If spraying is delayed and the grasshoppers advance to adult stages, they will be able to fly across the field and the whole field may have to be sprayed. A slight exception may be for two-striped grasshopper adults. Spraying field margins and roadside ditches can be effective for this species as the heavy-bodied adults do not fly very far and tend to walk into the crop from the field edges.
Application Timing
Grasshoppers should be sprayed when they are actively feeding, which is typically in the morning once temperatures have reached 20ºC. After the sun rises, grasshoppers will spend two to three hours basking in the sunlight to warm up. Once they are warm enough, they will begin walking around and start feeding. The majority of daily feeding occurs in the morning so this time of day is an ideal opportunity for spraying. On hot days, once temperatures begin to approach 30ºC, the grasshoppers will stop feeding and move down into the canopy where they rest vertically on plants to avoid the heat of the day. When temperatures cool down in the late afternoon, the grasshoppers will resume feeding and other activities such as mating or egg-laying. The afternoon feeding period is not as long as the morning period but still provides a second opportunity for an insecticide application during the day. Feeding starts to taper off under 18ºC and stops around 15ºC. Grasshoppers will again bask in the last few hours of sunlight before settling into their nighttime positions – typically on the ground for migratory and Packard’s species, and on plants in the upper half of the canopy for two-striped grasshoppers.
Ideal weather conditions for grasshopper feeding, and therefore spraying, are warm, clear, sunny days with no wind. Cloudy, cool, rainy, and windy weather all deter grasshopper activity and spraying will not be as effective. Higher insecticide rates may be required under less-than-ideal weather conditions. Traditional insecticides work best between 15 and 25ºC; this temperature window coincides fairly well with the temperatures favoured by grasshoppers for feeding. The efficacy of some synthetic pyrethroid products is reduced at temperatures greater than 25ºC.
Product Options
Registered insecticide options are listed in Table 2. Refer to individual labels for further details on crop usage, rates, and other application information.
The majority of insecticides enter the insects primarily through contact and must be sprayed directly on the grasshoppers in order to kill them. Thorough coverage is key for contact insecticides and higher water volumes may be needed for the product to penetrate dense plant canopies in order to reach the insects. Some contact insecticides also have ingestion properties allowing the grasshoppers to take in the insecticide through feeding on treated plant material. However, these products only stay on the surface of the plant tissue that they land on, so a rain shower or even a heavy dew can wash the insecticide off the plant. This residual control only lasts for up to a few days, and should be viewed as ‘bonus control’, not as certain as control by direct contact. Chlorpyrifos also has inhalation properties where the insecticide gasses off in the canopy, allowing the insect to ‘breathe’ it in through its spiracles. Again, this kind of residual control may only last up to 48 hours.
Chlorantraniliprole is a relatively new active ingredient with a different mode of action – Group 28. It is available as a single active in Coragen® and Coragen® MaX. This active ingredient works through ingestion of treated plant material and causes muscle paralysis in the insect; feeding quickly comes to a stop in as little as seven minutes. The grasshopper will become lethargic and die, although complete death may take up to a few days after ingestion. When applying Coragen®, the goal is to thoroughly cover the plant material that the grasshoppers are feeding on, rather than contacting the grasshoppers themselves. Coragen® has translaminar movement, moving from one side of a leaf to the other side, allowing for true residual properties. This residual control lasts between 7 and 21 days, depending on the rate applied. Chlorantraniliprole is not systemic, so it will not move into areas of new growth on the plant. The temperature when applying Coragen® also will not affect product performance. Because the product is being applied on the crop rather than on the insect, spraying can occur at any time of day, without having to wait until the grasshoppers are actively feeding. Coragen® can be applied in temperatures up to 40ºC, as long as coverage can be maintained. Coragen® is also highly selective on the insects that it has an effect on and has minimal impact on many important pollinators (bees) or other beneficial insects, compared to the non-selective properties of contact insecticides. That said, it is in best practice to avoid spraying when pollinators are actively foraging, to reduce unnecessary exposure.
Eco Bran is a grasshopper bait made with the active ingredient carbaryl in a wheat bran carrier. In order for this product to control grasshoppers, it must be ingested as it has no contact properties. Eco Bran provides up to 21 days residual control, depending on the size of the grasshoppers and population density (more and larger grasshoppers will consume more bait).
Nolo Bait™ is a biological insecticide containing spores of the parasite Paranosema (Nosema) locustae on a wheat bran carrier. It is registered for suppression of grasshoppers and must be ingested by the insects. The parasite infects the fat bodies of the insect, leading to sickness and death after 3 to 6 weeks. Infected grasshoppers can pass the parasite on to healthy grasshoppers and the parasite can remain within the grasshopper population for several years. Nolo Bait™ is most effective on younger grasshoppers (3rd instar).
Table 3: Insecticide Options Registered for Grasshopper Control in Pulse Crops
| Active Ingredient | Trade Name | Mode of Action & Mode of Entry | Pulse Crops Registered for Use | Pre-harvest Interval (PHI) (days) | Comments |
|---|---|---|---|---|---|
| chlorantraniliprole | Coragen®, Coragen® MaX | Group 28, Diamides, Ingestion | Pea, lentil, chickpea, faba bean, soybean, dry bean | 1 | Coragen® MaX is a 3x more concentrated formulation of Coragen® |
| chlorantraniliprole + lambda-cyhalothrin | Voliam Xpress® | Group 28, Diamides, Ingestion + Group 3A, Pyrethroids, Contact/ Ingestion | Pea, lentil, chickpea, faba bean, soybean, dry bean | 14 for pea, lentil, chickpea, faba bean, dry bean; 21 for soybean | |
| chlorpyrifos | Lorsban™, Pyrinex®, Nufos®, Citadel®, Warhawk®, Sharphos | Group 1B, Organophosphates, Contact/ Ingestion/ Inhalation | Lentil | 21 for rates up to 170 g ai/ac (420 g ai/ha); 60 for rates greater than 170 g ai/ac (420 g ai/ha) | Chlorpyrifos is being phased out. Retail sales permitted until December 2022; 2023 growing season is the last permitted application period. Check with grain buyer before applying to crop. |
| deltamethrin | Decis®, Poleci®, FBN Deltamethrin®, Advantage Deltamethrin® | Group 3A, Pyrethroids, Contact/ Ingestion | Pea, lentil, chickpea, faba bean, dry bean (Decis®); Lentil only (Poleci®, FBN & Advantage Deltamethrin®) | 7 (Decis®); 30 (Poleci®, FBN & Advantage Deltamethrin®) | Not all deltamethrin products are registered for all pulses; consult individual product labels for usage on specific crops. |
| malathion | Malathion 85E, Malathion 500 | Group 1B, Organophosphates, Contact | Lentil | 14 (Malathion 85E); 30 (Malathion 500) | |
| carbaryl wheat bran bait | Eco Bran | Group 1A, Carbamates, Ingestion | Dry bean | 5 | Also registered for application to field edges and roadsides (no PHI). |
| Paranosema (Nosema) locustae | Nolo Bait™ | Biological insecticide | All crops | 0 | Suppression of grasshoppers. Apply before flowering or post-harvest. |
Pre-harvest Interval
Strict attention must be paid to the pre-harvest interval (PHI) for the individual product being used on a specific pulse crop. The PHI is defined as the number of days between the pesticide application and harvest, which is the cutting of the plant by swathing or straight- cutting. Failure to adhere to the PHI guidelines could mean higher detectable amounts of insecticide residues in the harvested seed, which can cause shipments to be rejected by end-use customers and markets potentially lost. When choosing an insecticide for grasshoppers, always check the PHI requirements, especially if applying later in the growing season with harvest approaching.
Cultural Control
Seeding Date
Older plants are able to tolerate more grasshopper feeding than younger plants because of their higher amount of vegetative growth.
Earlier seeding helps to establish the crop sooner in the growing season. Earlier seeding also translates to earlier maturity in the growing season, and more mature crops are less attractive to the larger nymphs and adults that may be migrating to greener areas as harvest approaches.
Crop Establishment
An overall well-established crop will be able to withstand feeding damage more than a crop with poor establishment. Pay attention to seeding rate and seeding depth; the more plants that are successfully established means that the feeding done by the grasshopper population will be more diluted, with less damage on a per plant basis. Using seed treatments can help manage seedling diseases to lessen seedling mortality. Early season weed control from a pre-seed burnoff and/or soil residual herbicides will also increase the competitiveness of the crop.
Tillage
The purpose of using tillage for grasshopper management is to remove green plants – the food source for young nymphs. When nymphs hatch, they are tiny and cannot travel very far so they need to feed on whatever plants are available around them. If they cannot find food in their vicinity, they will starve. Therefore, if tillage is a suitable operation for the farm, it should be completed in early spring before the grasshoppers hatch, or at the very latest, when the grasshoppers have just started to hatch. Once the majority of nymphs have hatched, the benefits of tillage are greatly reduced. When performed successfully, tillage can reduce nymphal survival in the field quite significantly in some regions.
Tillage for the sole purpose of destroying grasshopper eggs is not advised due to the increased evaporation loss of soil moisture right before seeding and the potential for wind and water erosion of topsoil. There is also the chance that a large amount of grasshopper eggs may not even be directly in the field being tilled but could be in areas surrounding the field (roadsides, ditches, fence lines, etc.) that cannot be tilled. For this same reason, it is also unlikely that fall or spring fertilizer banding applications, such as anhydrous ammonia, have much of an impact on grasshopper populations. In addition, the chances of directly hitting the eggs with equipment would be minimal, especially if using narrow openers.
Crop Rotation
In general, using crop rotation as a grasshopper management strategy has a limited effect. The grasshopper species that feed on pulse crops also feed on a number of other crops (cereals, flax, canola, etc.), so rotating out of a pulse crop in lieu of another crop will not be more advantageous for population control. The grasshopper population is determined by the number and location of the eggs laid the previous fall, and the newly hatched nymphs will feed on whatever crop is available to them the following spring. Some pulse crops, such as peas, are less desirable than other pulse crops, but they will still get fed on if the grasshoppers need to eat. If there were fields with very high grasshopper numbers the previous year, then perhaps substituting a crop with less sensitivity than lentils may be beneficial. However, if the entire area was experiencing a grasshopper outbreak the previous year, there could be high populations in the overall geographical area the current year, so changing the crop planted on individual fields may not make a difference in terms of control.
Natural Enemies

Source: Jennifer Bogdan
Because grasshoppers are native insects on the Prairies, there are a lot of natural enemies already widespread across the province. These natural enemies can be as important as weather in keeping localized grasshopper populations in check. The spectrum of natural enemies collectively attack every stage of the grasshopper’s life cycle.
Egg Predators and Parasites
Important predators of grasshopper eggs include bee flies, blister beetles, ground beetles, and crickets. Females of certain species of bee flies and blister beetles lay their eggs in grasshopper egg- laying sites. These eggs develop and hatch quickly, and the larvae can consume large numbers of grasshopper eggs which develop more slowly. Some studies have shown bee fly and blister beetle larvae can destroy an average of 15 to 25%, even upwards of 60%, of grasshopper eggs. When ground beetle larvae are added to the predator mix, grasshopper egg destruction can be as high as 80 to 90% in localized areas. It should be noted that for blister beetles, it is the larvae of Epicauta species that feed on grasshopper eggs, not the colourful, metallic Nuttall’s blister beetle (Lytta nuttalli) whose larvae feed on the eggs and stored food in the nests of solitary and leaf- cutter bees (Figure 7).
Parasitic wasps of the genus Scelio parasitize grasshopper eggs soon after they are laid and can destroy between 5 and 40% of the eggs.
Nymph and Adult Predators, Parasites, and Diseases

Source: Jennifer Bogdan
Many different animals will eat grasshopper nymphs and adults, including spiders, gophers, coyotes, and birds. Native bird species such as the lark bunting, western meadowlark, sparrow hawk, and sharp-tailed grouse are large consumers of grasshoppers. Of most important is the lark bunting who eat mostly invertebrates; grasshoppers alone make up 85% of the diet they feed to their young and their nest numbers have been directly correlated to the grasshopper density in that area.
Parasitic flies produce maggots that feed inside the grasshopper before killing their host when they exit the body. Threadworms can also kill grasshoppers by growing inside their hosts after the grasshopper eats a threadworm egg.
The fungus, Entomophaga grylli, can build up to epidemic levels in grasshopper populations, typically under warm, humid conditions. Right before death, infected grasshoppers will climb to the top of the plant, wrap their legs around the stem and die. Fungal spores are released from the dead grasshopper that then infect other grasshoppers, and the cycle continues (Figure 8).
The parasite, Paranosema (Nosema) locustae, can reduce grasshopper populations by up to 60% in one year. Grasshoppers that eat infected vegetation or a dead, diseased grasshopper can become infected. This parasite also causes a reduction in the amount of eggs laid by infected females. Paranosema (Nosema) locustae is ubiquitous in the Prairie environment and is also commercially available as the product Nolo Bait™.
Key Messages
- There are three grasshopper species that are pests in pulse crops – the migratory, the two-striped, and Packard’s grasshoppers. These species belong to the spur-throated grasshoppers, identified by a spine or spur between their front legs. Grasshoppers that fly before June, have coloured hind wings, and/or sing or make clacking sounds when they fly are not pest species.
- Adult pest grasshopper species lay their eggs in late summer and fall. Eggs overwinter and hatch in the spring. Nymphs pass through 5 instar stages before becoming adults. Adults are able to fly and reproduce.
- Weather has a large influence on the grasshopper population for the following year. Cool, wet weather reduces egg-laying activity whereas a warm, open fall increases the amount of eggs laid. Cool, cloudy, and rainy weather slows grasshopper development during the growing season and decreases feeding activity. Grasshoppers prefer warm and dry conditions for growth, development, and reproduction.
- Conditions that favour lush crop development will increase the crop’s tolerance to grasshopper feeding. Grasshoppers prefer warm, open areas compared to the cool, humid conditions present under dense canopies.
- Grasshoppers actively feed when temperatures are between 21 and 27ºC, under clear skies with little to no wind. The majority of daily feeding takes place in the morning.
- Egg hatching typically begins in late May. Grasshoppers are often concentrated at field margins where they move into the crop from egg-laying sites (ditches, roadsides, fence lines).
- Lentils are the most sensitive pulse crop as grasshoppers avoid the foliage and prefer to feed on the flowers and developing pods. Grasshoppers can also clip the pods directly off the plants. The economic threshold for lentils is 2 grasshoppers per m2 at flowering and podding stages.
- For lentils, scout for nymphs in June in areas bordering lentil fields and then scout again during flowering and podding.
- Crop damage is the main factor when deciding to spray. If young nymphs are not eating a lot of foliage, consider monitoring the population as a lot can happen to grasshoppers between the nymph stages and a flowering and podding lentil crop.
- Grasshoppers are most easily controlled at the 3rd to 4th instar when nymphs are smaller, if populations are high and action is required. Higher rates of insecticide will be required for larger nymphs and adults, heavy populations, dense canopies, and less than ideal weather conditions. Adults are the most difficult to control due to their large size and their wider distribution across the field due to their ability to fly.
- Perimeter spraying of nymphs concentrated around the field edges is often enough to manage grasshopper populations and minimize economic damage.
- Contact insecticides should be applied to maximize direct contact with the grasshoppers in order for the best efficacy. These products should be sprayed when grasshoppers are actively feeding, which is determined by the weather.
- Chlorantraniliprole works by the grasshoppers ingesting treated plant material, so applications of Coragen® and Coragen® MaX are focused on thoroughly covering the crop rather than directly spraying the insects. Chlorantraniliprole also has translaminar movement, moving from one side of a leaf to the other (but not systemic or moving into new growth), thereby providing true residual control. Coragen® and Coragen® MaX are targeted, flexible, residual insecticide options that also have a favourable environmental and toxicological profile.
- Pay close attention to the pre-harvest interval of the insecticide chosen to not jeopardize pulse crop end-use markets.
- Natural enemies such as bee flies, blister beetles, ground beetles, birds, parasitic flies and wasps, and fungal diseases play a large role in keeping grasshopper populations in check.
Appendix 1: Grasshopper Identification
Two-striped Grasshopper (Melanoplus bivattatus)






Photo Credit: Dan Johnson, Lethbridge, Alberta
Migratory Grasshopper (Melanoplus sanguinipes) and Packard’s Grasshopper (Melanoplus packardii)






Photo Credit: Dan Johnson, Lethbridge, Alberta