By Saskatchewan Pulse Growers (SPG), Saskatchewan Canola Development Commission (SaskCanola), and Saskatchewan Wheat Development Commission (SaskWheat)
Drought Conditions Increase Herbicide Carryover Risk
Herbicide carryover becomes a concern after dry conditions because soil moisture largely dictates the rate of soil residual herbicide breakdown due to its influence on two of the major degradation mechanisms: microbial activity and chemical hydrolysis.
Although soil parameters such as pH and organic matter can influence degradation pathways, soil moisture is often the dominant factor. Soil microbes are most active in moist soils (50-100% field capacity), so a decrease in microbial activity in response to dry soil conditions reduces the amount of herbicide degradation that would typically be expected in the same time frame. Degradation through hydrolysis is also highly influenced by soil moisture as water breaks down herbicides by dividing the larger molecules into smaller, less active pieces.
The effect of drought conditions on soil temperature also influences the rate of herbicide degradation via microbial activity and chemical hydrolysis. Microbial activity is also dependent on soil temperature. It generally increases in warmer soils (up to 30°C) and decreases in cold soils (below 10°C). Therefore, in-season (June 1 to August 31) accumulation of precipitation is often used to inform risk assessments of herbicide carryover and guide re-cropping restrictions as the bulk of degradation through microbial activity will happen during these months with anticipated drop-off once soils cool down in September and into the fall.
Herbicide degradation via chemical hydrolysis will also occur more slowly at lower soil temperatures but continues to increase linearly with soil temperature even past a point of inhibition to microbial activity (see Figure 1). Although microbial degradation is often the greatest contributor to herbicide breakdown, chemical hydrolysis may partially compensate for lower amounts of microbial activity during a drought where soil temperatures may be high.
Drought conditions may also influence the soil solution concentration and favour a temporal increase in the concentration of H+ ions in the soil solution (i.e., a decrease in pH). Depending on the specific herbicide’s ability to persist under a given pH range, this effect may increase or decrease the degree of herbicide degradation via chemical hydrolysis.

Source: Adapted from Persistence of Herbicides in Soil. Penn State University.
1 Cessna, A. J., Knight, J. D., Ngombe, D., and Wolf T. M. (2017). Effect of temperature on the dissipation of seven herbicides in a biobed matrix. Canadian Journal of Soil Science
Increased levels of degradation via photolysis pathways may also occur under drought conditions through increased temperatures (indirect photolysis) or increased sunlight exposure (direct photolysis). It is important to note that photolysis is generally considered a minor mechanism of degradation for soil residual herbicides, and it should not be expected that photolysis would override impacts of major degradation pathways such as through microbial degradation or chemical hydrolysis.
“Soil microbes thrive in warm, moist soils, which results in faster degradation. It is estimated that there is a two to three-fold increase in chemical half-life with a 10°C decrease in temperature and a one and one-half to two-fold increase in chemical half-life if soil moisture content is reduced by a factor of two.”
— Eric Johnson, University of Saskatchewan, Factors Affecting Herbicide Residue: Impact of a dry year
Factors That Influence Herbicide Carryover
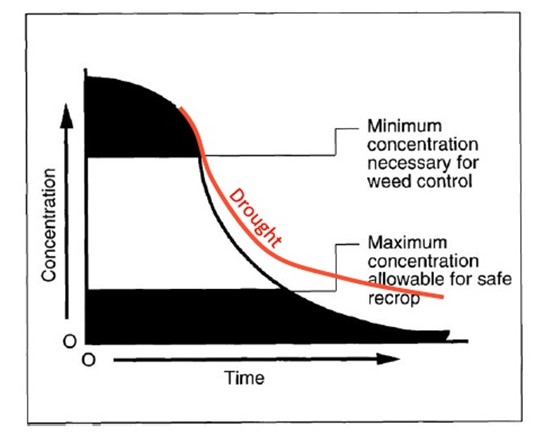
Source: Adapted from Persistence of Herbicides in Soil. Penn State University.
The degradation of soil residual herbicides occurs over time. Some soil residual herbicides may have rapid initial degradation. This is why some medium tolerant crops can be grown relatively quickly after herbicide application. The degradation curve may slow down with some active ingredients, requiring a longer period of time before residues are low enough to grow sensitive crops. For example, clopyralid (Lontrel™) requires at least two years between application and seeding of a pulse crop. In dry years, degradation is even slower and can cause unexpected crop damage.
Herbicide residues in the soil are deactivated in various ways including:
- Break down by soil microbes (most common method of degradation)
- Break down by chemical hydrolysis (water breaks herbicide molecules into less active pieces)
- Escape to the atmosphere as a gas (volatilization)
- Break down by light (photo degradation)
- Binding to soil particles
Microbial degradation is responsible for the majority of herbicide breakdown. Soil moisture and temperature are by far the most important factors in microbial activity.
Moisture: Moisture is the most important factor impacting herbicide breakdown, with microbial activity highest under moist but not saturated soils. Under dry or drought conditions, herbicide breakdown by soil microbes is reduced and carryover into the next growing season can occur. Herbicide adsorption to soil particles may also be increased with dry conditions. Additionally, breakdown by chemical hydrolysis is slower when rainfall and soil moisture are limited during the growing season.
Temperature: Optimum soil microbial activity occurs in June, July, and August when soil temperatures are warm between 20-30°C. Outside of this time frame, little herbicide breakdown occurs from soil microbes with minimal breakdown below soil temperatures of 10°C. Herbicides that are broken down by chemical hydrolysis can also slow down as chemical reactions occur more slowly at lower temperatures.
Soil texture and organic matter: Herbicide degradation decreases as soil organic matter decreases, due to lower water holding capacity and less microbial activity. Soils with low clay content have decreased adsorption of residual herbicides, which increases the potential availability of the herbicide to sensitive plant roots when a significant rainfall occurs. Potential for injury on subsequent sensitive crops increases as organic matter and clay content decrease.
Soil pH: Chemical hydrolysis and microbial degradation of residual herbicides within Groups 2, 5, 14, and 15 are influenced by soil pH.
- Group 2 imidazolinones (IMI) persist longer under acid (low pH) soil conditions, whereas sulfonylureas (SU) persist longer in high pH soils
- Group 2 herbicide flucarbazone-sodium (Everest®) has increased persistence under high pH of 8 or more
- Group 5 triazines degrade slower under high pH soils
- Group 14 sulfentrazone (Authority®) dissipates faster in high pH soils
- Group 15 pyroxasulfone (found in Focus® + Zidua® + Fierce®) dissipates faster in high pH soils
Degradation is not uniform within a specific field due to landscape variability. Soil characteristics such as soil texture, soil organic matter, pH, soil temperature, and soil moisture impact how each specific active ingredient degrades (Table 1) and can be highly variable within a field and between neighbouring fields.
Table 1: Soil Factors That Affect the Degradation of Some Residual Herbicides
| Group No. | Herbicides | Mechanisms of Degradation | Rate of Degradation is Slowed When |
|---|---|---|---|
| 2 | Imazethapy, Imazamox, Imazamethabenz, Imazapyr | Microbial | Soil pH < 7.0 Drought |
| 2 | Metsulfuron | Microbial/Chemical hydrolysis | Soil pH > 7.0 Drought |
| 2 | Flucarbazone | Microbial | Soil pH > 8.0 Drought |
| 3 | Trifluralin, Ethafluralin | Microbial | Drought |
| 4 | Clopyralid | Microbial | Drought |
| 4 | Halauxifen | Microbial | Drought |
| 4 | Quinclorac | Microbial | Drought |
| 14 | sulfentrazone | Microbial | Drought; High soil pH; Low CEC |
| 14 | flumioxazin | Microbial | Drought |
| 15 | pyroxasulfone | Microbial | Drought; High soil pH; Low CEC |
| 27 | pyrasulfotole | Microbial | Drought |
Source: Factors Affecting Herbicide Residue: Impact of a dry year. Eric Johnson, University of Saskatchewan.
Impact of Herbicide Carryover
Potential yield reductions associated with herbicide carryover injury are difficult to predict. Although there have been studies to assess yield impacts on sensitive crops, results may only be relevant to the specific environmental conditions during the period of research. Some products may not have been tested in environmental conditions as extreme as the province has experienced over the last several years. Environmental extremes may result in more crop damage, but favourable environmental conditions during the season of injury may also help crops withstand the stress of herbicide carryover injury and allow for a certain extent of crop recovery.
Evaluating Herbicide Carryover Risk
Precipitation
In-season precipitation accumulated after herbicide application generally provides the most reliable indicator of potential risk associated with herbicide carryover. Herbicide carryover risk maps generated by the Saskatchewan Ministry of Agriculture or publicly accessible weather station data provide guidelines for regions potentially at increased risk for herbicide carryover. However, localized field precipitation records are often more beneficial in assessing individual field risk because precipitation events within a season may be sporadic and variable across a relatively small distance.
Soil Testing
It is possible to have soil samples sent to a lab to be tested for chemical residues. However, this process is expensive and beyond confirmation (that the herbicide is present), cannot provide sufficient information to accurately predict if crop injury will result in response to levels extracted by test. Just as detection does not guarantee injury, certain herbicides may be active and initiate a crop injury response below the level of detection by the laboratory analysis. Soil testing for herbicide residues does not account for the interactions of the herbicide with soil properties; therefore, it cannot be used as an indicator of the bioavailability of that herbicide.
Residual carryover can also be highly variable across the field due to differences in soil properties at different landscape positions, and these differences cannot be represented in the lab setting with traditional extraction methods.
Soil testing can also provide additional information to assess herbicide carryover risk by revealing different soil properties that affect herbicide breakdown within a field. Analysis of soil texture, soil organic matter, and pH can help identify relative risk between fields if soil
characteristics are categorically different across regions of the farm.
Because individual fields cannot be considered a homogenous unit, site-specific soil sampling protocols may be needed to better capture variability across the field. Unless two fields distinctly represent two contrasting soil types, it will be difficult to use a single soil sample to represent the characteristics of the entire field. Even then, general soil testing can help inform the relative risk of herbicide carryover, but cannot predict it and its extent entirely. Areas with higher organic matter are generally at a lower risk for herbicide carryover.
Soil organic matter acts as a buffer by increasing binding capacity for herbicides in the soil, keeping them away from plant roots. Additionally, higher organic matter soils generally have higher microbial and better water holding capacity activity to assist with herbicide breakdown.
Soil texture can also be used to determine herbicide carryover risk. Lighter, or sandier soils, have less binding or adsorption capacity, which significantly increases the risk of herbicide carryover. Herbicide carryover risk increases as organic matter and clay content decrease. Soil pH can also be used to identify herbicide carryover risk.
Bioassay
Field bioassays are often recommended on herbicide labels as a method of evaluating possible herbicide carryover tolerance of sensitive rotational crops. These in-field test strips provide a preview of the rotational crop’s growth and development in the field with suspected herbicide carryover. Because landscape variability impacts herbicide carryover, field bioassays need to be large enough to indicate crop response representative of the entire field. Insights gained from field bioassays are generally not timely enough to inform a cropping decision until the second season after application.
Plant bioassays can be used as a shortcut to growing rotational crops out in the field. This method brings the soil from the field into a greenhouse and grows out susceptible plants in pots to evaluate if they will be impacted by herbicide carryover. Similar to soil testing for residue levels, plant bioassays may not be a reliable indicator of crop response in the field due to soil sampling limitations and under-representation of field variability.
A control, with soil collected from or strips planted into an untreated area of the field, should ideally be included in both bioassay methods as it provides a reference for subtle differences in visual injury. However, this is not always possible and presents a major limitation for using them to consistently predict injury. Bioassays can help provide insights into the impact of herbicide residues present in the soil; however, neither methodology is foolproof nor should they be used in isolation to make re-cropping decisions.
Table 2: Re-cropping Restrictions for Residual Herbicides
| PRODUCT | Faba beans | Dry beans | Field Peas | Lentils | Soybeans |
|---|---|---|---|---|---|
| 2,4-D* | 1 | 1 | 1 | ||
| Altitude FX3 | 1 | 1 | |||
| Amitrol 240 | 10d* | 5d* | 1 | 6d | |
| AAtrex, Primextra II Magnum | 1* | 1* | |||
| Ares | 1 | 1 | |||
| Assert (Black and Grey Wooded soils) | 1 | ||||
| Assert (Brown and Dark Brown soils) | 2 | ||||
| Authority 480◊ | 0 | 0 | 2 | 0 | |
| Authority Strike◊ | 0 | 0 | 2 | 0 | |
| Authority Supreme | 0 | 2 | 0 | ||
| Clomazone, Command Charge, IPCO Trigon | 1 | 1 | 1 | 0 | |
| Clopyralid/MCPA (+/- fluroxypyr) | 2 | 2 | 1* | 2 | 2 |
| Dicamba* | 1* | 1 | |||
| Dicamba/Fluroxypyr | 2 | 2 | 1 | 1 | 2 |
| Eclipse, Clopyralid | 10 mths* | ||||
| Ethalfluralin | 0 | 0 | 0 | 0 | 0 |
| Express Pro | 10 mths | 10 mths | 10 mths | 10 mths | 10 mths |
| Fierce | 0 | ||||
| Flextstar GT | 10 mths | 10 mths | |||
| Florasulam | 1 | 1* | 1 | 1 | 1 |
| Florasulam/fluroxypyr + MCPA | 1 | ||||
| Florasulam + glyphosate (prior to Aug 1) | 1 | ||||
| Flucarbazone (Brown soils) | |||||
| Flucarbazone (Dark Brown soils) | 1* | 1 | |||
| Flucarbazone (Black soils) | 1 | 1* | 1 | ||
| Flucarbazone (Grey-Wooded soils) | 1* | ||||
| Fluroxypyr, Sentrallas | 1*** | 1*** | 1 | 1 | 1*** |
| Focus | 0 | 0 | 0 | ||
| Frontier Max | 0* | ||||
| Heat Complete | 1 | 0 | 0 | 0 | 0 |
| Imazamox/Imazethapyr*, Odyssey Ultra Q* | 1 | 1††† | 1 | ||
| Imazethapyr | 0 | 1 | |||
| Imazamox, Imazamox+bentazon, Image | 1 | 1 | 1 | ||
| Infinity / Tundra / Velocity m3 | 1 | 2 | 1 | ||
| Kerb SC | 1 | 1 | 1 | 1 | 1 |
| Korrex | 1 | 1 | 1 | 1 | |
| Metolachlor | 1 | ||||
| Metribuzin | 0 | 0 | 0* | ||
| Metsulfuron (pH less than 7, Brown and Dark Brown | 3 | ||||
| Metsulfuron (pH less than 7, other soils) | 3 | ||||
| Metsulfuron (pH 7 to 7.9, Brown and Dark Brown soils) | 4 | ||||
| Metsulfuron (pH 7 to 7.9, other soils) | 4 | ||||
| Muster | 2 | 2 | 2 | 2 | |
| Nicosulfuron | 10 mths | ||||
| Option | 1 | 1 | 1 | ||
| Paradigm PRE, Exhilarate | 1 | 1 | 1 | 2 | 1 |
| Permit WG | 0 | 1 | 1 | ||
| Pixxaro | 1 | 2 | 1 | ||
| Quinclorac | 1 | 2 | |||
| Reflex* | 0 | 1 | |||
| Rexade | 1 | 2 | 1 | ||
| Rimsulfuron, Sortan IS | 1 | 1 | 1 | 1 | 1 |
| Signal FSU | 1 | 1 | |||
| Simplicity | 1 | 1 | 1 | ||
| Smoulder | 11 mths | 11††† mths | 11 mths | ||
| Tandem | 1 | 1 | |||
| Thifen:triben (2:1) + fluroxypyr | 2 | 2 | 1 | 1 | 2 |
| Thifen:triben (25:25) + fluroxypyr | 1 | 1 | 1 | 1 | 1 |
| Travallas | 10 mths | 10 mths | 10 mths | 10 mths | |
| Trifluralin | 0 | 0 | 0 | 0 | 0 |
| Triallate | 1 | 1 | 0 | 1 | 1 |
| Triallate/trifluralin | 0 | 0 | 1 | ||
| Valtera (Crop uses) | 1 | 9 mths | 0 | 1 | 0 |
| Varro, Predicade | 1 | 1 | 1 | 1 | |
| Voraxor , Voraxor Complete | 1 | 1 | 1 | 1 |
The minimum re-cropping intervals are listed. These intervals may be longer than those listed depending on the use rates, region, province, soil types, environment,
time of application and crop variety. Refer to product page for more information.
** Drought restrictions apply to drought conditions (80% of normal June to September rainfall) for high pH soils (greater than pH 7.5) and severe drought (less than 65%
of normal June to Sept. rainfall) for all soils.
*** May not be supported for all products; see product page for details.
† May not be valid for all varieties or crop types. See product page for details.
††† Clearfield lentils ONLY.
◊ Replant intervals are for high rate
0 – May be seeded or reseeded the year of application. No re-cropping restrictions. 1 – Next cropping season after application. 2 – Two cropping seasons after application.
NR – Not recommended.
Note: The re-cropping intervals listed may not be sufficient to prevent crop injury during periods of below average rainfall.
Source: Adapted from the Guide to Crop Protection, published by Saskatchewan Ministry of Agriculture.
Mitigating Herbicide Carryover Risk
Crop Selection
Crop rotation serves as the most significant tool to mitigate risk in fields anticipating a high level of herbicide carryover. Although large changes to a crop rotation can be a challenge, it is important to consider substituting highly sensitive crops for lower-risk alternatives that still have agronomic suitability.
For instance, pulse crops have the highest tolerance to imidazoline-based herbicide residues and chickpeas, faba beans, field peas, and lentils can all be grown safely the year following imidazoline application according to labels. However, care should be taken to avoid back-to- back seasons of pulses to mitigate risks associated with disease, soil erosion, and weed management.

After pulse crops, cereals exhibit the next best tolerance to residual carryover of imidazoline- based herbicides. However, tolerance among cereal species is not equal as spring wheat is substantially more tolerant to imidazoline residues compared to oats, Canary seed, durum, and barley. Oilseed crops are generally the most sensitive to imidazoline herbicide injury, and among them, mustard and canola rank the highest in sensitivity, followed by flax.
With the exception of tolerance gained through CL® crops, differences in varietal tolerance within a specific crop type have not been identified.
Agronomic Practice
Crop injury caused by the carryover of residual herbicides can be further aggravated by other abiotic and biotic stresses imposed on the crop. Aside from rotation, there is no single agronomic intervention that can fully eliminate herbicide injury. However, following the best management practices for the crop to ensure its vigorous establishment and growth remains an important recommendation to lessen additional stress factors imposed on the crop.
Increasing seeding rates to achieve higher than optimal plant population targets is not recommended because most active ingredients associated with residual herbicide injury do not act as a germination inhibitor. In fact, herbicide carryover injuries are most likely to reveal themselves after a rainfall event that allows an active ingredient to be washed into the root zone once it has been desorbed from soil particles. Although it is still important to target optimal plant populations for specific crops, higher stand densities will not increase a crop’s tolerance to herbicide residues and may result in a higher number of injured plants field wide.
Delayed seeding may be another strategy to help buffer negative crop responses associated with herbicide carryover. Establishment in cold soils can be stressful on crops, and a reduced growth rate is often observed, which can exacerbate the impacts of herbicide carryover injury. Furthermore, delays in seeding to allow for crops to germinate in warm soils can also provide a wider window for herbicide degradation, especially if moisture from spring snowmelt or rainfall has been received and microbial activity is increasing in response to improvements in soil moisture and temperature. Due to cool soils in the spring, herbicide breakdown will be negligible until soils are warmer than 5-7°C.
Although delayed seeding can be helpful, it is not a standalone strategy that can guarantee full protection against injury associated with herbicide carryover. There is no precise recommendation for how much additional time or moisture is needed in the spring if in-season herbicide degradation was expected to be below average.
Compounding Herbicide Carryover
CL® crops (i.e., lentil or wheat) can safely be re-cropped on fields with Imidazoline (Imi) herbicide carryover. However, when growing CL® tolerant crops on land that has been impacted by Imi herbicide carryover, the crop should be managed conventionally without the use of Imi herbicides in-crop. Applying Imi products, such as Ares™ on CL® tolerant crops is common practice. However, in the case of carryover, it can lead to crop damage and a further increase of herbicide residual in the soil.
Research conducted in the early 2000s found that under some environmental conditions “back- to-back” application of residual Group 2 herbicides could result in additive or synergistic injury. This can occur with persistent group 2 herbicides such as imazethapyr and metsulfuron. This is known as herbicide stacking. Some Group 2 residual herbicides can accumulate in the soil over multiple growing seasons, if herbicide breakdown is slowed, and result in significant damages to sensitive crops. It should be noted that not all Group 2 herbicides have the same residual properties.
Small amounts of different residual herbicides can accumulate over several growing seasons to contribute to the injury of a sensitive crop. Areas where drier conditions have been observed over multiple seasons are at a higher risk for herbicide stacking, which occurs when multiple applications of residual herbicides from the same group are sprayed in rotation. Group 2 herbicides are more likely to carryover at levels that can damage plants because they can maintain herbicidal activity at low doses.
There are areas of the province that have seen repeated drought for more than one year. These areas in particular need to be aware of possible carryover from previous years and take herbicides applied in the past into consideration when planning for next year. To see these areas, view Saskatchewan Agriculture’s Herbicide Carryover Risk Maps.
Key Takeaways
- Herbicides are primarily broken down by adequate moisture, temperature, and time. Most of Saskatchewan did not meet these conditions. There are other factors that play a small role in herbicide breakdown.
- Some herbicides are known to pose a greater carryover risk than others, but many have not been tested under conditions reflective of the environmental extremes of the 2021 season and subsequent dry years after.
- Group 2 herbicides in the Imidazoline family, such as imazamox and imazethapyr, pose a high risk for herbicide carryover. Imazamox and imazethapyr are found in herbicides commonly sprayed on pulses and CL® tolerant crops. Solo®, Odyssey®, Viper®, Davai®, Python®, Quasar®, and Ares™ are all examples of herbicides that contain imazamox, imazethapyr, imazapyr or a combination. See product labels or product manufacturers for more details. Be aware of potentially sensitive re-cropping options such as durum wheat, canary seed, and canola. Some chemical manufacturers have made label changes following the extreme heat and drought of 2021.
- Tools are available to help determine herbicide carryover risk, but they must be used together, not independently of each other.
- There are ways to help decrease herbicide carryover risk. Crop rotation, herbicide rotations and certain agronomic practices can help but will not completely eliminate risk.