Research Experts: Dr. Michelle Hubbard (Agriculture and Agri-Food Canada [AAFC]), Dr. Zakir Hossain (AAFC), and Dr. Bruce Gossen (AAFC)
Summary Prepared by Alexis Adams MSc PAg
Introduction
Anthracnose, caused by the fungus Colletotrichum lentis (C. lentis), is the primary disease affecting lentils in Western Canada. Symptoms of anthracnose include white, grey, or cream-coloured spots on leaves and similar-coloured lesions on stems. Pin-shaped black microsclerotia also frequently develop in the stem lesions in an irregular pattern. The symptoms lead to premature leaf drop, girdling of stems, lodging of plants, and yield loss, with complete plant death in severe cases. Ascochyta is another common disease in lentils that can be distinguished from Anthracnose by the dark margins that develop around disease spots and the concentric circle pattern formed by pycnidia within disease spots. Stemphylium can also be confused with Ascochyta. However, Stemphylium often starts as small, light beige lesions on leaves that spread to take over an entire leaflet or even a branch.
Fungicide Groups 3 (demethylation inhibitors), 7 (succinate dehydrogenase inhibitors), and 11 (strobilurins) are the main active ingredients (a.i.) in commercial fungicides registered to control anthracnose disease in lentil crops. Insensitivity to these fungicide groups can occur with continual use over time. Insensitivity to Group 11 fungicides was first observed in the field in 2019 due to a single-site mutation in the C. lentis pathogen cytochrome b gene. Due to the highly site-specific activity of strobilurins, insensitivity to one Group 11 a.i. renders C. lentis insensitive to all Group 11 a.i. Relative to Group 11, the risk of fungi developing insensitivity to Groups 3 and 7 fungicides is moderate. Also, the development of insensitivity to one Group 3 or 7 product does not necessarily mean insensitivity to other products in the same group.
The objective of this research project was to assess the extent of C. lentis insensitivity to Group 11 fungicides through a widespread survey of anthracnose in lentil fields in Saskatchewan, followed by a molecular assessment of fungicide insensitivity. The project also included a preliminary screening for insensitivity to Groups 3 and 7 fungicides. Agronomic and environmental factors, such as lentil variety and landscape position, were analyzed to explore the interactions between anthracnose severity and strobilurin insensitivity.

Research Methodology
Field Survey
Surveys were conducted in 130 lentil fields in 2020, 29 fields in 2021, and 61 fields in 2022. Ten sites per field were sampled in a W pattern in 2020, with the number of sample sites reduced to five sites per field in 2021 and 2022. Sample slope position was noted as low point, mid-slope, flat, or high point. At each sample site, ten plants were rated for anthracnose incidence and severity. Incidence was rated as the proportion of the ten plants at each sampling site that exhibited anthracnose symptoms on a scale from 0–1. The severity of the ten plants sampled was rated on a scale from 0–9 in 2020, which was simplified to a scale from 0–5 for 2021 and 2022. The criteria for the 0–5 scale are as follows:
- 0: No lesions
- 1: < 5% of stems and leaves in the ten plants are diseased; disease occurs only in the lower canopy, with a few superficial lesions at the stem base.
- 2: 5-25% of the stems and leaves rated in the ten plants are diseased; the disease occurs only in the lower canopy, with lesions and some leaf drop observed.
- 3: 25-50% of the stems and leaves in the ten plants rated are diseased; the disease occurs in the lower and mid-canopy, with lesions and leaf drop observed.
- 4: 50-75% of the stems and leaves in the ten plants rated are diseased; the disease occurs in the lower, mid, and upper canopy, with lesions, leaf drop, and shoot die-back observed.
- 5: > 75% of stems and leaves in the ten plants rated are diseased; disease occurs at all canopy levels, with lesions, leaf drop, and severe shoot die-back observed.
The severity and incidence of anthracnose were compared among lentil varieties, fungicide application, landscape position, and damage from hail or insects. Agronomic data collected from sample sites varied and included location, crop rotation, herbicides, and drought stress. After collection, samples were sent to the pulse pathology laboratory at the Swift Current Research and Development Centre, Agriculture and Agri-Food Canada (AAFC).
Assessment of Strobilurin Insensitivity
C. lentis was isolated from lentil tissue exhibiting anthracnose symptoms as a resource for this and future research. DNA was extracted from air-dried above-ground plant tissue exhibiting anthracnose symptoms. Two polymerase chain reaction (PCR) assays were performed to determine whether the sample contained strobilurin-sensitive C. lentis, strobilurin-insensitive C. lentis, both, or neither. The assay results were compared to a control, which was a DNA isolation from C. lentis isolates known to be sensitive or insensitive, provided by BASF.
Growth Chamber Experiments
Growth chamber experiments were initiated to validate the PCR results. If the PCR analysis were valid, plants inoculated with a C. lentis isolate that showed Group 11 insensitivity in the PCR assay would still develop anthracnose after a Group 11 strobilurin application in the greenhouse. Conversely, a plant inoculated with C. lentis that was sensitive in the PCR assay would not develop the disease on plants treated with a Group 11 fungicide. Unfortunately, the isolates inoculated onto the plants did not consistently produce spores at a high enough level for anthracnose to develop and for the treatments to be compared. Many different strategies to induce sporulation were employed before researchers moved on to other methods of validating the PCR results.
In vitro Assessments
Because the growth chamber experiments were unsuccessful, in vitro tolerance assessments were conducted to validate the PCR assays for strobilurin insensitivity and to perform a preliminary assessment of succinate dehydrogenase inhibitor (Group 7) and demethylation inhibitor (Group 3) sensitivity. C. lentis isolates were grown on agar plates amended with various concentrations of the fungicide a.i. under analysis, as well as unamended agar plates for the untreated control. Radial mycelial growth on the agar plates was assessed for each treatment to determine C. lentis sensitivity.
For the Group 11 assessment, agar plates were prepared with 0.5, 1.0, 5.0, and 10 mg/L of trifloxystrobin in 2021, and with 1.00, 1.87, 3.74, or 7.48 mg/L of trifloxystrobin in 2022. These plates were inoculated with either a sensitive or an insensitive C. lentis isolate.
For the Group 7 assessment, benzovindiflupyr and fluopyram were amended onto agar plates at varying concentrations in an initial dose-response experiment. Next, 14 old baseline C. lentis isolates were compared to 27 new isolates from the 2020 and 2021 surveys. The old baseline isolates were from Dr. Bruce Gossen’s lab and were collected before the use of Group 3 and 7 fungicides in the field. Therefore, C. lentis would not have had the opportunity to develop insensitivity to these a.i. All isolates were grown on plates amended with 0, 0.01, or 0.1 mg/L benzovindiflupyr or 0 or 35 mg/L fluopyram. Radial growth was measured after 12 days.
Similarly, for the Group 3 assessment, an initial dose-response experiment was conducted for propiconazole and prothioconazole, followed by an isolate comparison. After this, 14 old, 21 new, and two control isolates were amended on plates with 0.03 or 0.09 mg/L of prothioconazole or propiconazole. Radial growth was compared after 13 days.
Results and Discussion
Anthracnose Severity and Incidence from Lentil Field Survey
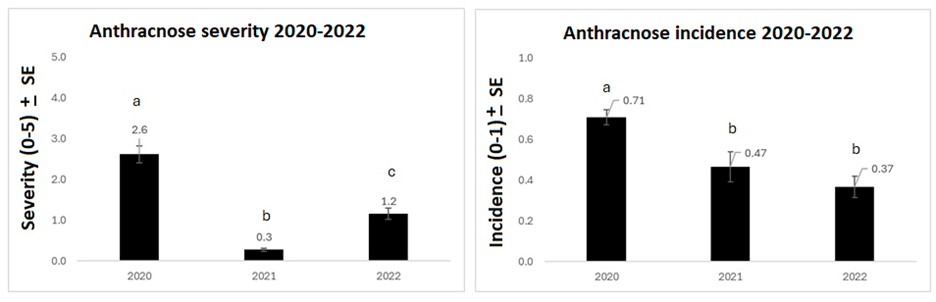
Average disease severity ratings from the lentil field survey were 2.6 in 2020, 0.3 in 2021, and 1.2 in 2022, on a 0–5 scale. Differences between each year were statistically significant. Anthracnose had the lowest severity rating in the driest year, 2021, indicating an expected connection between disease severity and field moisture conditions. Anthracnose incidence was 0.71 in 2020 (71% of plants had developed anthracnose), 0.47 in 2021, and 0.37 in 2022, with 2020 being significantly higher than 2021 and 2022 (Figure 1). 2020 was the worst year for anthracnose incidence and severity.
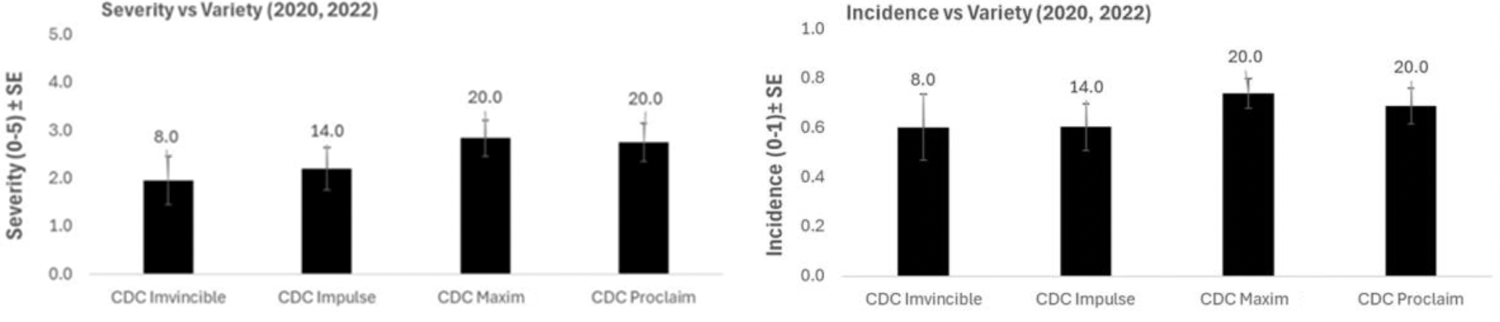
Disease severity and incidence were not different between the most common lentil varieties (Figure 2). This is likely because most lentil plants are bred with resistance to the less virulent race of anthracnose, race 1, but not to the more virulent race 0. Race 0 is the likely cause of most anthracnose in the field.
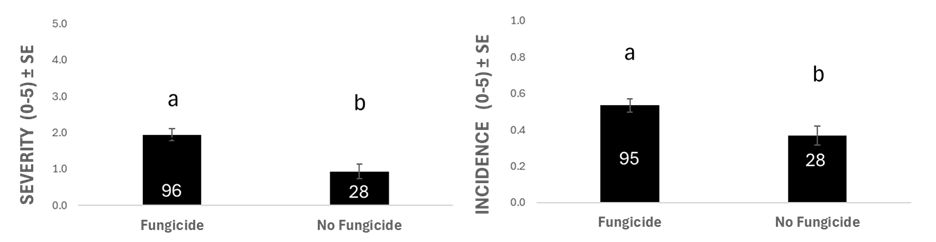
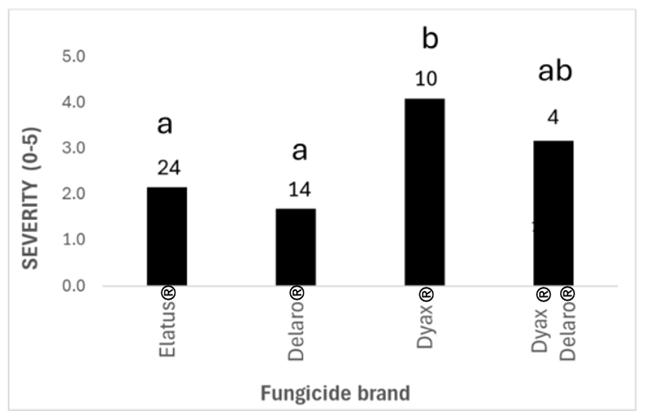
Surveyed fields where fungicide had been applied had significantly higher anthracnose incidence and severity (Figure 3). Higher anthracnose ratings do not indicate that the fungicide was not effective on the disease. Instead, it may suggest that increased disease pressure led farmers to apply fungicide. When analyzing the specific fungicide products used, lentils sprayed with Dyax® (fluxapyroxad, Group 7; pyraclostrobin, Group 11) had higher disease severity than those sprayed with Delaro® (prothioconazole, Group 3; trifloxystrobin, Group 11) or Elatus® (benzovindiflupyr, Group 7; azoxystrobin, Group 11) (Figure 4). As stated above, this result may be because farmers chose this fungicide when disease pressure was higher. After all, they felt it worked better. However, it may indicate that Dyax® is less effective or that C. lentis is developing insensitivity to the Group 7 a.i. in Dyax®, fluxapyroxad. Further investigation and controlled field trials are necessary to properly compare the impact of these different fungicide products on disease severity.
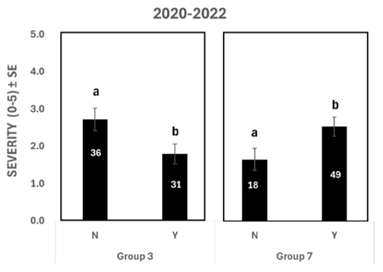
When comparing the application of Group 3 and Group 7, disease was significantly less severe in fields where Group 3 was applied and more severe in fields where Group 7 was used for the combined years of 2020 and 2022 (Figure 5).Other growth chamber research funded by Saskatchewan Pulse Growers and completed by Insight Plant Health Corp. showed that when the C. lentis fungus is insensitive to strobilurins, Group 3 actives were more effective than Group 7 actives at reducing anthracnose severity. However, the sample size for the survey is too small and variable to conclude fungicide effectiveness across lentil-growing regions in Saskatchewan. Growers should pay attention to fungicide performance on their farms.
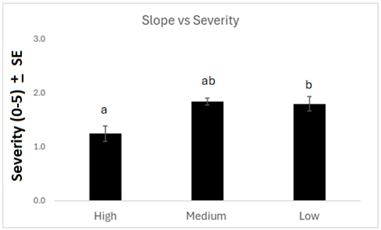
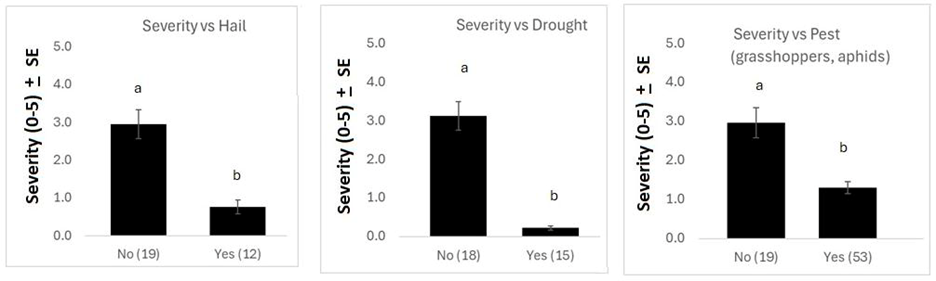
Examining other agronomic factors, there was no difference in anthracnose incidence between landscape positions when all years were combined. However, severity was significantly greater at low field points than high points (Figure 6). This result is logical, as low field positions are typically higher-producing and have a thicker crop canopy, which could increase disease pressure and subsequent severity. Fields with hail damage, insect damage, or drought stress had less severe anthracnose than fields with no damage (Figure 7). This may be because the crop damage or stress caused reduced stand density, leading to less humid conditions and thus reduced disease pressure.
Molecular Assessment Results
Group 11 Insensitivity
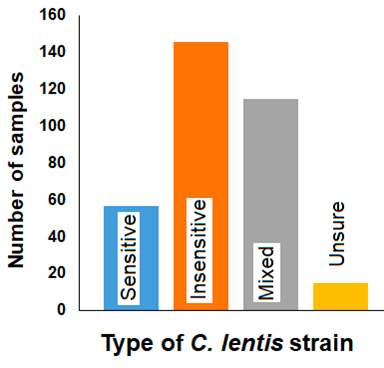
In 2020, a total of 333 samples underwent PCR analysis. The results showed that 44% of samples were strobilurin-insensitive, 35% had a mix of sensitive and insensitive isolates, 17% of samples were sensitive, and 5% of samples could not be typed (Figure 8). Many fields contained a mix of sensitivity results among samples, indicating that certain parts of a field may be infected with C. lentis that is strobilurin-insensitive. In contrast, other parts may still be sensitive to Group 11 fungicides. Growers may see mixed responses to strobilurin fungicide application across a field, but should expect strobilurin insensitivity in at least some parts of the field. The results from the in vitro assessment are generally consistent with those of the PCR analysis.
In 2021, due to dry conditions, only 20 samples with anthracnose symptoms were collected. Of those samples, the PCR analysis showed 30% sensitive, 15% insensitive, and 55% mixed sensitive and insensitive isolates. The sensitivity to strobilurin was higher in 2021 than in 2020, at 17%. In 2022, 127 samples were analyzed, and 19% of samples were sensitive, 26% were insensitive, and 55% had mixed sensitive and insensitive C. lentis isolates.
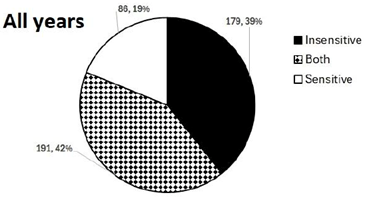
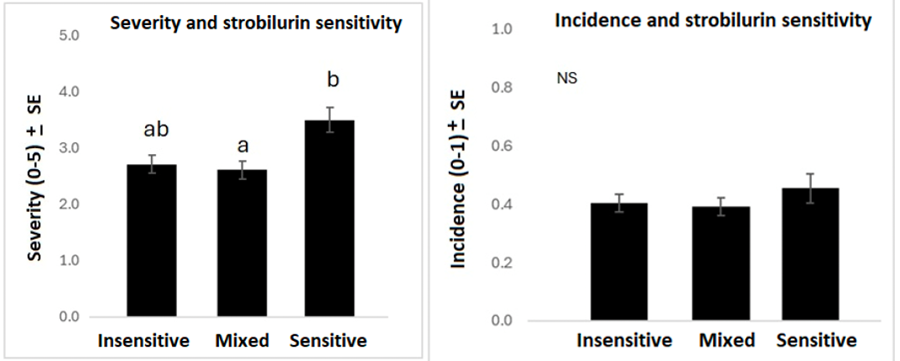
With all years combined, 39% of field sample sites contained insensitive-only isolates, 19% of sites were sensitive only, and 42% of sites included a mix of sensitive and insensitive C. lentis strains (Figure 9). Disease severity was statistically higher at sites where only strobilurin-sensitive C. lentis strains were detected compared to sites with both insensitive and sensitive C. lentis (Figure 10). Additionally, the number of insensitive sites was highest in 2020, when the disease was most severe, and the lowest proportion of insensitive sites was observed in 2021, when the disease was least severe. However, there is no clear evidence from this survey that insensitivity is increasing rapidly. Data from more years with similar moisture conditions would be necessary to determine a rate of change in insensitivity.
Looking at agronomic factors collected in the survey, with all years combined, the proportion of sensitive sites decreased at lower landscape positions, with 19% sensitive at low, 22% at medium, and 27% at high positions. This result suggests that farmers may experience slightly less disease control when applying strobilurin products in low spots in the field.
Overall, C. lentis insensitivity to strobilurins is widespread, as most fields assessed showed at least partial strobilurin insensitivity. Growers should not depend solely on Group 11 a.i. for anthracnose control. In years when disease severity is higher, growers can expect strobilurin insensitivity to be even greater, and should consider applying fungicides with Group 3 and 7 actives if possible.
Group 7 and 3 in vitro Insensitivity Assessments
There was no evidence of resistance development to Group 3 or 7 fungicides from the in vitro assessments. Radial growth of both the old baseline and the new C. lentis isolates decreased with increasing concentrations of the Group 7 a.i. benzovindiflupyr and fluopyram. See Figure 11 for an example of the radial growth of in vitro isolates. The older isolates generally grew more slowly than the new isolates across both a.i. However, with the high dose of benzovindiflupyr, the new isolates exhibited slower growth, indicating an increase in sensitivity rather than the expected decrease in sensitivity (Figure 12). In Group 3 in vitro assessment, growth was similar between old and new C. lentis isolates for both propiconazole and prothioconazole, indicating no insensitivity development to either Group 3 active.
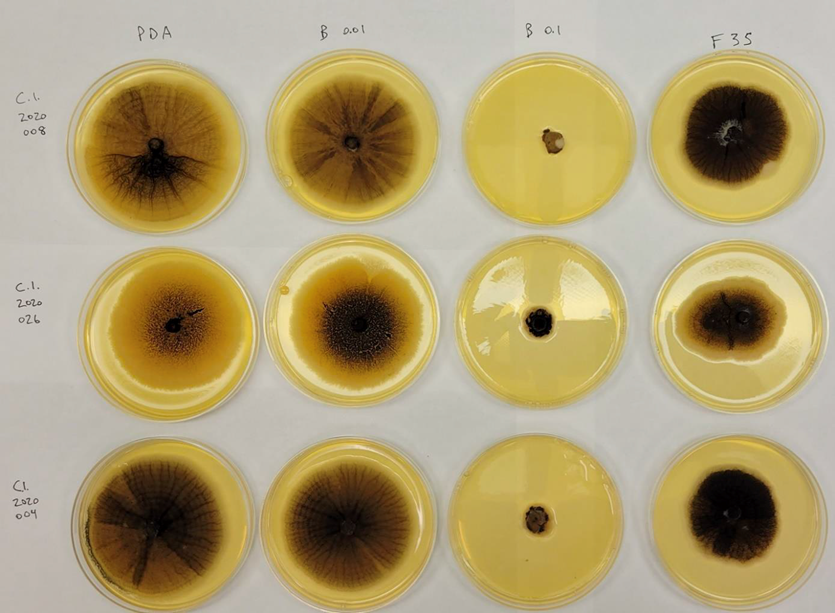
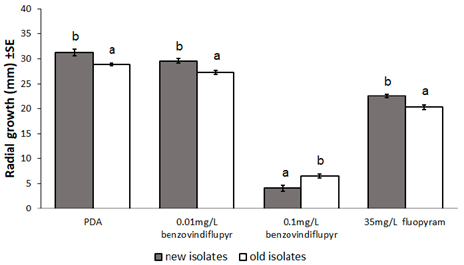
Conclusions and Recommendations
Most lentil fields exhibit some incidence of Group 11 strobilurin insensitivity, although parts of the field may contain C. lentis fungus that remains sensitive to strobilurins. Consequently, farmers and agronomists may achieve anthracnose control with strobilurins in parts of the field, but should not expect complete control in any field. The incidence of strobilurin insensitivity did not increase over the three survey years, indicating that insensitivity is not spreading rapidly. Growers should expect to see more strobilurin insensitivity in years with higher disease pressure and should choose a fungicide with multiple modes of action beyond Group 11. Based on the survey results and in vitro assessment, resistance is not developing to Group 3 and 7 a.i. At this time, they are still effective for reducing anthracnose incidence and severity in lentils. For more information, refer to the Saskatchewan Pulse Growers’ guide to lentil fungicide options.



