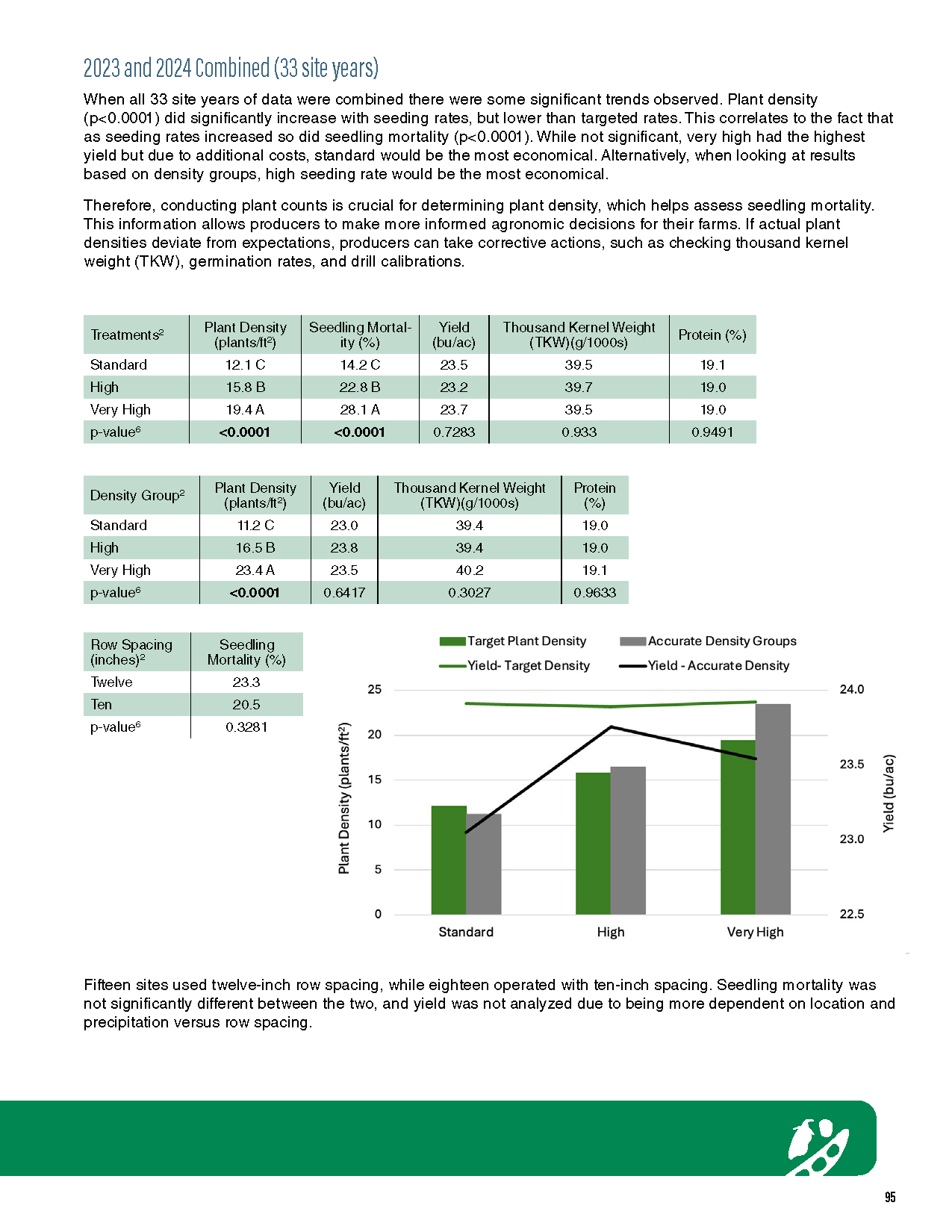
Seed is the foundation of every crop and the best way to determine its quality is through a simple seed test. Without a seed test, once a seed issue is realized, significant costs will have been incurred and yield potential of that crop or from re-seeding another crop will have declined. Environmental conditions in the year the seed is grown impact its quality. Seed grown and harvested in a drought year can have significant quality impacts, just as seed grown in a wet year. Furthermore, seed quality can change after long periods of storage and, therefore, seed testing is a meaningful investment and planning tool ahead of every season.
Seed Test Interpretation
Germination
Germination describes the percent of seed that are likely to germinate under optimal and standardized oxygen, light, moisture and temperature conditions. Germination is an accredited test; cereals undergo a brief cold period to break dormancy and are then tested at 20°C for seven days. There is no pre-chill requirement in chickpea, lentil or pea crops but temperature is also held at 20°C with final counts performed at seven days later. At the end of this test period, seeds are evaluated and placed into various germination categories including fresh, hard, abnormal, and dead seeds.
- Fresh seeds fail to germinate. These seeds will imbibe water regularly and initially appear to be capable of germination but then remain dormant. Fresh seeds may be viable, but cannot be confirmed.
- Hard seeds fail to germinate. The seed coats of hard seeds will not be penetrated by water and remain intact at the end of the test period.
- Abnormal seeds germinate, but do not have adequate plant structures to maintain healthy growth such as missing roots or shoots.
- Dead seeds cannot produce any part of a seedling. They may imbibe water and crack the seed coat but will die before they will produce seedling. Higher levels of seed-borne disease usually correlate to a higher percentage of dead seeds.
The germination test is useful, as it tells us the maximum potential of the seed lot under optimal conditions. This information can be used to determine if, under ideal growing conditions, a particular seed source should be planted at all. However, germination is only a single component of seed quality. When germination is poor, a different seed source may be selected. When germination is good, the decision to plant a seed lot should not be based on germination alone. Germination is largely influenced by late-season environmental conditions, and harvest weather that is too cold, too wet, too hot or too dry can all reduce germination.
Germination can also be negatively impacted by applications of pre-harvest glyphosate and therefore it is not recommended to plant seed that has been treated with pre-harvest applications of glyphosate as chemically damaged seed may show lower germination values, poor root development, and higher than acceptable levels of seedling mortality. Exceptional circumstances may result in a desire to test seed that has been treated with pre- harvest glyphosate that was applied at the proper timing. Seed labs can estimate germination of seed treated with pre-harvest glyphosate if the germination test is done in soil. Submitting a seed sample that received pre-harvest glyphosate will result in abnormal seedling development, inaccurate results, and the seed lab will have to redo the test, costing extra. The risks mentioned above are still valid, so it is recommended to source seed elsewhere instead of using treated with pre-harvest glyphosate.
Vigour
The vigour test measures a seed lot’s ability to produce normal seedlings under adverse conditions. Unlike the germination test that is ran under optimal conditions, vigour is most often measured using a cold stress test which evaluates the seedlings’ ability to withstand cool temperatures (5°C for seven days), and accompanying stress that may be typical of early spring planting. Vigour in a seed lot is important because it may provide insight into how seedlings will perform under challenging conditions. Seed lots with higher vigour may have faster and more uniform seedling emergence in low temperature soils or other stressful conditions.
Low vigour values may also provide an early warning of diminishing seed quality as seed lots will begin to drop in vigour before germination values start to fall. Conditions of seed development, maturation, storage, and seed aging all impact vigour. Seeds developed under moisture stress, nutrient deficiency, or extreme temperatures are often characterized by light, shriveled, and low- vigour seed. Similar to germination, vigour will also be negatively impacted by mechanical damage or improper storage.
The vigour test is not standardized among seed labs. While the cold stress test is common, the parameters and the stress used may vary between labs. Vigour tests from different labs should not be compared directly.
Seed-borne Pathogens
Fungal screening for seed-borne pathogens is an important component of assessing seed quality as these diseases can have negative impacts on crop establishment, overall plant health, and ultimately crop yield. Evaluation of seed-borne pathogens is particularly important for diseases that have high rates of seed- to-seedling transmission. For example, Ascochyta rabiei carried on chickpea seed is readily transmitted to the seedling and can be highly destructive to the crop. Therefore, only chickpea seed lots with low infection levels (<0.3%) are acceptable for planting. Even if this threshold is met, a seed treatment is still recommended as the likelihood of missing an infected seed is very high and even a very small percentage of Ascochyta-infected chickpea seed can result in significant seedling infection.
A chickpea seed lot with an Ascochyta infection of 0.1% (one infected seed in 1,000 seeds) and a planting density of three to four plants ft2 poses the risk of 175 infected chickpea seedlings per acre. Such a level of early infection for an aggressive disease like Ascochyta can have a devastating impact on chickpea crops.
400 seeds are evaluated in a chickpea disease screen for Ascochyta and, therefore, a single seed with Ascochyta would result in an infection of 0.25%.
Seed treatments are an excellent tool to help protect cereal and pulse seedlings against early-season soil and seed-borne pathogens. Seed treatment selection should be based on the targeted pathogens of concern and should be applied by means to ensure thorough seed coverage and according to label instructions. Some seed treatments may not be compatible with certain seed-applied rhizobium inoculants, so it is recommended to consult with manufacturers for compatibility information. It is important to note that seed treatments alone cannot compensate for a poor seed lot and some seed lots simply have too high of disease levels to be used as a seed source. Guidelines have been developed to help inform decisions of acceptable thresholds for seed-borne pathogens, but guidelines are only guidelines. Soil moisture and temperature conditions following planting will also influence what infection levels are tolerable in a given year or location. There are no hard rules on acceptable levels of disease due to each producer having a unique situation, and acceptable level of risk.
Thousand Kernel Weight
The thousand kernel weight (TKW) is a measure of seed size and represents the weight, in grams, of 1,000 seeds. Seed weight may also be reported, which is the weight of a single seed, in milligrams, and is numerically equivalent to TKW. Most crops have a typical TKW range but it is important to note that TKW is unique to each individual seed lot and can vary substantially between varieties, fields, and growing seasons. For instance, smaller and lighter seed with lower TKWs is often a consequence of a drought season when limited moisture resources did not allow for optimal seed fill.
Measuring the TKW of a seed lot is important for calculating the optimal seeding rates for pulse and cereal crops as it provides a more precise measurement of seeds sown compared to bushels or weight. Providing that all seed quality factors are equal between two seed lots, the seed source with the higher TKW will require a heavier seeding rate to achieve the same optimal plant stand. This is because the bigger the seed, the higher the TKW and the fewer seeds per pound. Therefore, for the same weight (i.e. 60 pounds) of seed, fewer potential plants are being put in the ground.
Table 1. Guidelines for seed-borne disease thresholds in cereal and pulse crops(1)
| Major Pathogen | Threshold | Crop(s) | Comments |
|---|---|---|---|
| Cereals | |||
| Common Root Rot Cochliobolus sativus | N/A | Barley, Durum, Oats, Wheat | Thresholds indicate the level at or above which a fungicidal seed treatment is recommended. A fungicidal seed treatment may be warranted at lower levels if there are additional risk factors. Combined disease levels should be considered when considering seed treatment options. |
| Seedling Blight/Root Rot Fusarium spp. | 10% | Barley, Durum, Oats, Wheat | |
| Fusarium graminearum | 2%2 | Barley, Durum, Oats, Wheat | |
| Loose Smut Ustilago nuda | 2% | Barley | True loose smut in barley is specified in the Seeds Act with a maximum of 2% allowable. Loose smut requires a systemic seed treatment for control. |
| Pulses | |||
| Ascochyta spp. | 0.3% | Chickpeas | Seed-to-seedling transmission of Ascochyta rabiei is high in chickpea; seed with 0% should still always receive a seed treatment and seed with levels above 0.3% should not be used. |
| 10% | Field Peas | Up to 10% infection should not significantly affect establishment of field peas. | |
| Lentils | A seed treatment should be used if infection levels are close to or exceed 5% for lentils grown in Brown and Dark Brown soil zones. Lentil seed with levels exceeding 10% should not be planted at all. Any level of seed- borne Ascochyta spp. is unacceptable for lentils grown in Black soil zone. | ||
| Anthracnose Colletotrichum truncatum | N/A | Lentils | Seed-to-seedling transmission of anthracnose is very low in lentil and infected residue provides a greater inoculum risk; however, it is still important to plant clean seed and accept zero tolerance in being planted into a field that has never grown lentil. |
1Adapted from Saskatchewan Ministry of Agriculture guidelines
2Additional information on thresholds for Fusarium graminearum is available from the Saskatchewan Ministry of Agriculture
Optimal Plant Stands and Seeding Rate Calculations
Targeted Plant Population
Plant population is an important factor for the establishment of competitive, high-yielding cereal and pulse crops. Optimal plant stands differ by crop, environment, and management factors but care should be taken to target appropriate plant densities for each individual crop and farm operation.
Table 2. Summary of target plant populations for select cereal and pulse crops
| Crop | Plant Population Target (Range) plants ft-2 | Typical TKW Range (grams) |
|---|---|---|
| Cereals | ||
| Barley | 22 (20‒24) | 30‒50 |
| Durum | 20 (20‒24) | 41‒45 |
| Oat | 24 (20‒30)1 | 30‒45 |
| Wheat | 24 (20‒24) | 30‒40 |
| Pulses | ||
| Chickpea | 4 (3‒4) | 220‒425 |
| Field Pea | 8 (7‒9) | 125‒300 |
| Lentil | 12 (10‒17) | 25‒80 |
1While a plant population of 20-30 plants ft-2 is usually recommended, research has consistently shown the benefits of increased seeding rates, particularly in the presence of weeds such as wild oats. Next to early seeding, seeding rate is one of the most powerful strategies available for reducing weeds.
2Lentil populations greater than 12 plants/ft2 can reduce weed biomass and improve yield. Densities on the higher range may be risky for environments with increased disease pressure, but generally for moderate-low risk environments, plant stand targets of 17 plants/ft2 can be successful in combination with well-timed fungicide applications.
Calculating Seeding Rate
Once the desired plant population is determined the seeding rate can easily be calculated based on specific seed quality parameters of each individual seed lot. Although this calculation is fairly straightforward, the seedling survivability rate can be difficult to estimate and will vary among farms and across growing season. As a general rule of thumb for cereal and pulse crops, expected seedling survivability is approximately 5‒15% lower than the germination rate with a lower survivability rate anticipated under adverse growing conditions.
Factors to consider when assigning seedling survivability include:
- Seeding date
- Soil temperature, moisture, and texture
- Seed handling and seeding speed
- Seeding depth
- Seed-placed fertilizer
- Seedling pests such as insects and soil-borne pathogens
![Seeding rate (lbs/ac) = [(desired plant population in square feet) x (TKW in grams)]/[(seeling survival rate in decimal form)/(10.4)]](https://saskpulse.com/wp-content/uploads/2023/04/Seeding-rate-formula-1024x314.png)
Increased seeding rates will be required to overcome low seedling survivability; however, it is important to recognize that not all issues of poor quality seed can be corrected by increased seeding rates.
For instance, large differences in vigour and germination values in a seed lot can be exacerbated by increasing seeding rates which may impose greater variability in establishment and lead to a more uneven plant stand. Similarly, increasing seeding rate to overcome low germination of a seed lot with a high pathogen load will simply introduce more disease into the field.
Value of Seed Testing in a Dry Year
Seed grown during a dry year typically boasts lower disease levels relative to an average or wet season, but reductions in seed-borne pathogen load do not guarantee high quality seed. Particularly impacted are germination, vigour, and TKW with potential reductions in all categories.
- TKW: When seeds do not have adequate moisture resources during grain fill, final seed production is typically much lower than expected crop averages
- Vigour: Production of gibberellin hormone, responsible for regulating seed maturity and dormancy, is typically reduced under drought conditions and seed may demonstrate higher than anticipated levels of dormancy and lower vigour as a result. Grain, particularly pulses, harvested during very hot and dry conditions can be susceptible to micro-cracking on the seed coat leading to reductions in vigour before germination levels are noticeably affected.
- Germination: Mechanical damage from typical harvest and handling activities is often increased under hot and dry conditions, especially when seed moisture content is low and air temperatures are high. Such damage to the seed coat can have a significant negative impact on germination levels with pulse crops being the most prone to seed coat cracking and splitting.
Initial seed testing reports from the fall of 2021 are indicating low germination in some crops, particularly in pea. Pea samples experienced high levels of mechanical damage during harvest operations and some labs are reporting majority of pea samples with sub-optimal germination levels.
Be wary of sub-par germination and vigour values on post-harvest seed tests. Germination and vigour levels can continue to decrease over winter while being stored and are also likely to be further compromised with further seed coat damage induced through additional handling during storage transfer and cleaning. It is a recommended practice to get seed tested again in the spring for germination, vigour, and TKW.
Seed Test for Crop Success
Seed is arguably the most valuable input for any crop and ensuring its quality is of utmost importance, regardless of the season. Although interim seed survey results show historically low levels of mean pathogen infection and high proportions of disease-free seed, other seed quality parameters such as germination and vigour may have been compromised due to increased levels of mechanical damage during a season characterized by extended periods of extremely hot and dry conditions. Seed testing in the fall is a great way to evaluate the seed quality potential of the seed source on farm, but seed testing for germination, vigour, and TKW should also be repeated in the spring to ensure no significant changes have resulted after extended storage and further handling. Final seed test results should be used to fine-tune seeding rate calculations to target optimal plant stands and help get the crop off to the best start possible in the spring.