Jennifer Bogdan, PAg, CCA
Ascochyta blight is the most serious disease of chickpea and the largest obstacle in chickpea production for growers in Saskatchewan. Ascochyta can affect all growth stages of the plant (seedling, vegetative, flowering, podding) and can cause lesions on all parts of the plant (leaves, stems, pods, developing seeds). As a result, yield loss can be devastating; over 70% yield reduction can occur if the disease is not controlled. Ascochyta can also be detrimental to seed quality, resulting in lower grades.
Ascochyta blight of chickpea is caused by the pathogen Ascochyta rabiei which is a different, and much more aggressive, pathogen than the Ascochyta blight that affects lentils (Ascochyta lentis) or peas (Ascochyta pisi (leaf and pod spot)).
Disease Cycle and Symptoms
The pathogen overwinters on infected chickpea crop residue and seed. Infected seed can play an important role in both the introduction of A. rabiei to new areas, as well as in early disease development as the pathogen is easily transmitted from the seed to seedlings. Both asexual spores (conidia, spread by rain-splash) and sexual spores (ascospores, spread by wind) can be produced on the crop residue. In the late fall and early spring, sexual reproduction produces pseudothecia which house the ascospores; development of pseudothecia takes 5-7 weeks under adequate moisture and moderate temperatures (near 10ºC). In spring and early summer, mature pseudothecia release ascospores into the air which can travel for several miles to infect chickpea crops. In Saskatchewan, ascospores are assumed to be released even before the chickpea crop emerges. Airborne ascospores are thought to be the initial source of infection in the spring, although rain-splashed conidia may also be involved. It is also during sexual reproduction that genetic recombination occurs, which can increase the genetic diversity within the pathogen population. This makes it more difficult to breed for resistant chickpea varieties and also increases the chance of fungicide-resistant strains developing.
After the spore infection takes place, symptoms begin to develop within 4-6 days. Early lesions are tan to dark brown with a dark brown margin (Figure 1). Three to six days after lesions are formed, dark brown pycnidia develop; pycnidia are often arranged in concentric rings and will not rub off the tissue like debris will (Figure 2). Conidia ooze out of the pycnidia in a sticky spore mass and are spread by rain-splash onto healthy plant parts, causing new infections (Figure 3); the majority of the lesions during the growing season result from the rapid development of pycnidia and conidia under humid conditions (Figures 4-8). Even small rain showers are enough to spread conidia to new plant tissue. Ascochyta in chickpea is a poly cyclic disease, meaning that multiple infection cycles can occur throughout the growing season under adequate moisture and temperature (20-25ºC).

Source: Melanie Leppa, Soils & Such Agronomy

Source: Sabine Banniza, CDC, University of Saskatchewan

Source: Sabine Banniza, CDC, University of Saskatchewan

Source: Sabine Banniza, CDC, University of Saskatchewan

Source: Sabine Banniza, CDC, University of Saskatchewan

Source: Saskatchewan Ministry of Agriculture

Source: Sabine Banniza, CDC, University of Saskatchewan
Scouting
Due to the aggressive nature of ascochyta in chickpea, scouting needs to happen early and needs to happen often. Early identification of ascochyta allows for more timely decisions before the disease gets well established in the crop. Chickpea fields should be scouted individually as each field has a different field history, agronomic practices, and exposure to disease inoculum. Even if a field has never had chickpeas grown on it before, the wind-blown ascospores can travel from chickpea stubble fields miles away and infect new chickpea crops early in the season.

Source: Sabine Banniza, CDC, University of Saskatchewan
Scouting should start at the seedling stage, after the plants have emerged and rows are starting to form (approximately 2 to 3 weeks after seeding). Check 5 to 10 sites in the field in a “W” or large circular shape, paying attention to ‘hot spots’ (low spots or other areas of moisture accumulation, heavier crop canopy, headlands, damaged plants). Tall flags or GPS can be used to mark certain areas in the field to be revisited at each scouting event in order to check for new lesions and to evaluate the fungicide program; taking pictures on a mobile phone for a visual record can be useful here. Using a small spade, remove plants from the soil to closely inspect the leaflets and stems for small discoloured spots; check root health for signs of other diseases as well as nodulation. Rub any spots seen to remove debris; early lesions are very small (the size of a pinhead) so a magnifying glass may be useful for identification. Scout every 3 to 7 days during the seedling stage. If rain and/or humid conditions exist, scouting should be more frequent. After a fungicide application, scout within 7 days to reevaluate disease severity; if a fungicide was not applied, scout within 3 to 5 days for reassessment. Scouting should be continued until the pod filling stage.
Fungicide Application
Fungicides control disease by providing a protective barrier on or within the plant tissue that prevents disease spores from germinating and infecting the host tissue. Therefore, fungicides must be applied to plant tissue before spore infection takes place. Some fungicides also have limited curative properties that can stop disease development but only within the first 24 to 36 hours after spore infection (during the incubation period). A curative fungicide must be applied within 36 hours after a rainfall to be effective. Curative fungicides will not repair lesions that have already damaged the plant tissue. It is best to use fungicides in a preventative manner (i.e. prior to rainfall) for effective ascochyta control.
Product labels may refer to an active ingredient having “systemic” properties – this refers to the fungicide being able to move through the xylem in the plant (where water movement takes place) and the direction of movement is only upward or outward within the leaf. In contrast, with herbicides, the term “systemic” refers to movement in the phloem (where sugars are transported) and the active ingredient will move in all directions throughout the plant, including to roots and growing points (i.e. such as with glyphosate). Some groups of fungicides are contact only (Group M ) and will not penetrate the plant tissue, whereas other groups are systemic (Groups 3, 7, 11) and move into the tissue. Curative fungicides do have systemic properties, but not all systemic fungicides are curative. There can also be differences in the level of systemic activity between active ingredients within the same group, such as for Group 11 strobilurins.
Foliar fungicides only last for approximately 10-14 days within the plant; this timeframe is shortened under favourable growing conditions (good moisture and warm temperatures) or when a highly susceptible variety is grown.
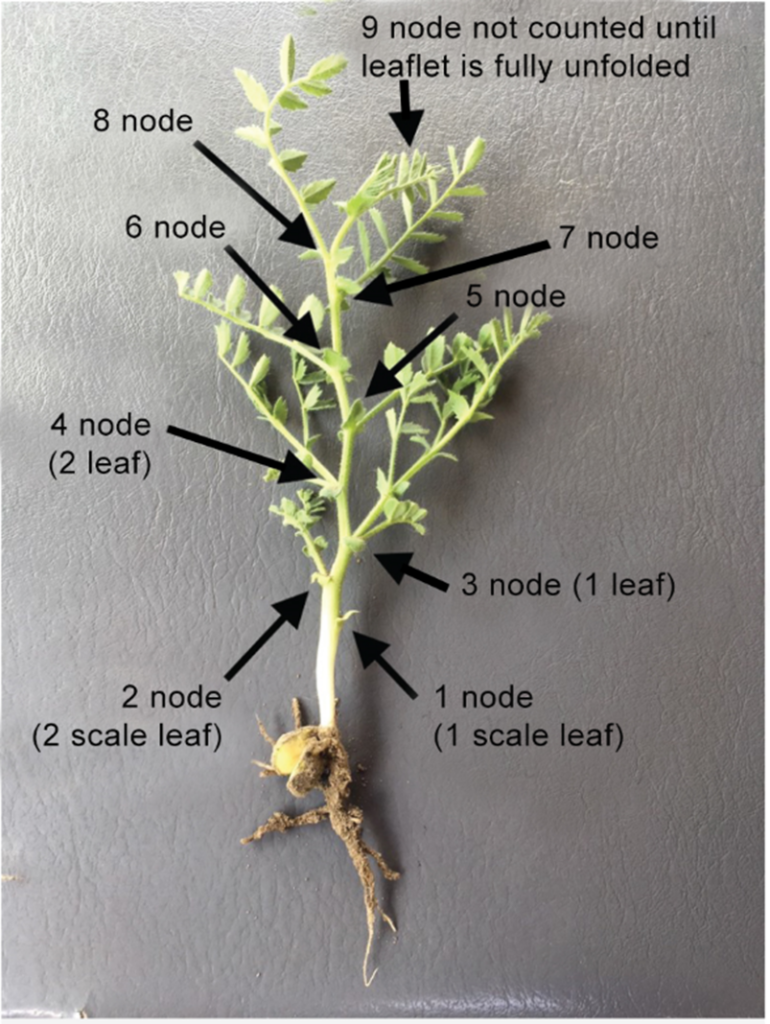
Timing and Coverage: The most important piece with fungicide application is to spray early, starting at the 7 to 10 node stage. Older information recommending the first application be at the start of flowering is outdated; experienced growers and agronomists agree that the first application should occur around the 8 node stage (Figure 9). While it may look like a lot of bare ground being sprayed at this stage, chickpeas will form a lush canopy that will fill in to create a humid microclimate for disease development to persist (Figure 10). Even if the conditions are drier when the crop stage is ready for the first application, do not delay this application until the crop is bigger – always make the first application as planned at the 7 to 10 node stage. If required, a second application may be made to protect the flowers, then make decisions on subsequent applications based on the weather forecast, disease progression (i.e. scouting), and crop stage.
In an average year with moderate rainfall, it is typical to require 4 fungicide applications to keep ascochyta managed throughout the growing season (Figures 11 & 12). In drier years, it is possible that only 2 applications are needed; in wetter years, as many as 5 applications may be required.
The quality of the application is even more important than the individual product chosen. Since fungicides are applied in a protective manner, coverage is critical for their performance. While lower water volumes (minimum 12 gallons per acre) can be used for the first application (around the 8 node stage), higher water volumes (20 gallons per acre) are necessary for the droplets to infiltrate the dense chickpea canopy in successive applications.
Fungicide Resistance Management: Strobilurin (Group 11) fungicides are one of the main groups used for ascochyta blight in chickpea. However, in previous years, the overuse of strobilurins (multiple, consecutive applications of products containing only this single mode of action during the growing season) resulted in the selection of strobilurin-resistant ascochyta isolates in Saskatchewan. In 2007, it was found that almost the entire A. rabiei population was resistant (insensitive) to strobilurins. In recent years, more fungicide options have become available and with this reduced dependence on strobilurins, it is possible that the overall level of resistance in the pathogen population has dropped. As a result, strobilurins may provide effective control on some isolates within the population. However, it is important that a single application of a strobilurin fungicide is not used alone in order to avoid putting selection pressure on the population, as the level of strobilurin resistance within the pathogen population can increase quickly. Therefore, proper fungicide stewardship is critical for ascochyta management as fungicide groups are limited for this disease; only Groups 3, 7, 11, and M are available, and Group 11 may have limited efficacy. The following guidelines are recommended (for more information, visit the Fungicide Resistance Action Committee at www.frac.info):
- Do not use a strobilurin alone;
- Use tank-mixes containing multiple modes of action (groups);
- If a strobilurin was used in one application, rotate to a non-strobilurin product for the next application;
- Do not apply the same group more than two times on the same field in one season (except for chlorothalonil, which can be applied three times);
- Do not use a strobilurin as the last application of the season in order to reduce the number of strobilurin-resistant isolates that will over-winter, cause disease in subsequent years, and increase in abundance under additional selection pressure.
Fungicide options: Newer chickpea fungicides all contain multiple modes of action (either Group 3 + 11 or Group 7 + 11), whereas other products may only contain a single mode of action (such as Group 3 and Group M) which can be used to rotate out of a strobilurin. Be aware of resistance management recommendations above when using a fungicide that contains only a strobilurin as their labels may not reflect the current fungicide resistance management recommendations; if using a strobilurin-only product, consult the manufacturer representative for a compatible tank-mix partner from a different group. Also, pay close attention to the maximum number of applications allowed per season as well as the pre-harvest interval for all products being considered.
Source: Melanie Leppa, Soils & Such Agronomy

Source: Melanie Leppa, Soils & Such Agronomy

Source: BASF
Economic Thresholds
The following factors should be considered to help decide when to stop applying fungicides:
Crop Stage
- Fungicides are not recommended past the first week in August as it takes approximately 1 month for a flower to develop into a fully-formed seed. New flowers produced in mid-August will not likely have time to form a mature seed before a fall frost in mid-September.
- Fungicides applied later in the season can keep the crop greener, especially if they contain a strobilurin product, and could also increase green seed content. Chickpeas are already a very indeterminate crop and days to maturity are critical to getting the crop in the bin. If a fungicide is needed for pod protection, a non-strobilurin option such as chlorothalonil is a good choice. After the crop begins to senesce, fungicides will not be beneficial.
Disease Severity or Crop Damage
- If disease control was ineffective and there are numerous pod lesions, a fungicide application likely will not be beneficial.
- If the crop has been injured from other means (hail, herbicide injury, etc) and maturity is delayed, the risk of fall frost must be considered – if the injury is not too severe and occurs at the vegetative stage, then a fungicide application to protect the crop is usually justified. However, if the injury is more severe and occurs at podding, then it likely would not be worth a fungicide application to protect the crop against new disease.
Crop Value
- The following equation can be used to calculate the economic threshold:
% Potential Yield Loss x Estimated Yield (lb/acre) x Price ($/lb) =
Economic Loss in Yield ($/acre)
If economic yield loss is less than fungicide application cost, do not apply a fungicide. If economic yield loss is more than fungicide application cost, then a fungicide is likely warranted.
Be sure to check with Crop Insurance if deciding to not spray and take a loss instead, as choosing to not follow disease management practices may affect any claims.
Integrated Pest Management Strategies
The following agronomic considerations should be used in conjunction with early fungicide application and diligent scouting to best prevent ascochyta in chickpea:
- Field Selection: Do not plant chickpeas next to a chickpea stubble field. Wind-blown ascospores in the spring from the previous year’s chickpea crop can easily infect neighbouring chickpea crops in the current year. Choose a field at least 500 m away from chickpea stubble and wait at least 2 years before planting chickpeas directly beside a chickpea stubble field.
- Chickpea Class: Kabuli chickpeas are more susceptible to ascochyta than Desi chickpeas.
- Variety Resistance: Choose varieties with a high level of resistance. Ascochyta ratings for each variety are available in the seed guides
- Seed Quality: Seed should be tested for ascochyta presence as seed to seedling transmission with this pathogen is high. Ideally, use seed with 0% ascochyta; do not use seed that has over 0.3% Ascochyta rabiei.
- Seed Treatment: The use of a seed treatment on Kabuli chickpeas is standard practice with experienced growers for a few reasons. First, seed testing is only performed on a small number of seeds that represent a seedlot and even if the seed test comes back at less than 0.3% A. rabiei, there is still a chance that other untested seeds could be infected. Second, Kabuli chickpeas have a thin, low-tannin seed coat, making these varieties completely susceptible to seed rot and seedling blight caused by pythium; Desi chickpeas have a thick, dark-coloured seed coat containing tannins which provide an anti-fungal effect against pythium (although they can still be infected by other seed and seedling diseases).
- Seeding Rate: Use the Thousand Seed Weight calculation to achieve optimum plant stand densities. Target 38 to 44 established plants/m2 (3 to 3.3 established plants/ft2) for Kabuli chickpeas and 44 to 50 plants/m2 (3.3 to 4.5 established plants/ft2) for Desi chickpeas. Seeding higher plant densities above the recommended rate with more susceptible varieties may increase disease severity.
- Field Rolling: Rolling fields after the chickpeas have emerged can damage the plants due to their stiff stems. Weakening or injuring the plants can cause them to become more susceptible to disease infections. If rolling is necessary, it should be completed prior to crop emergence.



